Abstract
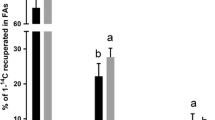
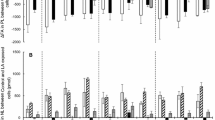
In vitro cultivated Atlantic salmon (Salmo salar L.), hepatocytes were incubated without or with a mixture of sesamin and episesamin in order to test for possible effects on lipid metabolism. Sesamin/episesamin exposure (0.05 mM, final concentration) led to increased elongation and desaturation of 14C 18:3n-3 to docosahexaenoic acid (14C 22:6n-3, DHA, P < 0.01) and down regulated gene expression of Δ6 and Δ5 desaturases compared to control treatment. Sesamin/episesamin further increased the hepatocytes capacity for fatty acid β-oxidation of 14C 18:3n-3 (P < 0.01) to the 14C acid soluble products, acetate, malate and oxaloacetate, in agreement with an increased gene expression of carnitine palmitoyltransferase I. Also the gene expression of cluster of differentiation 36 was upregulated and the expression of scavenger receptor type B, peroxisome proliferator-activated receptors α and γ were downregulated. The amount of triacylglycerols secreted by the cells tended to be lower in the sesamin/episesamin incubated hepatocytes than the control cells. This study shows that sesamin has favourable effects on lipid metabolism leading to increased level of DHA, which may be of interest for aquaculture use.





Similar content being viewed by others
Abbreviations
- ACO:
-
Acyl-CoA oxidase
- ASP:
-
Acid soluble products
- BSA:
-
Bovine serum albumin
- cd 36:
-
Cluster of differentiation 36
- CPT1:
-
Carnitin palmitoyl transferase I
- DHA:
-
Docosahexaenoic acid (22:6n-3)
- EDTA:
-
Ethylenediaminetetraacetic acid
- EF1A:
-
Elongation factor 1α
- EPA:
-
Eicosapentaenoic acid (20:5n-3)
- FAD:
-
Flavin-adenine-dinucleotide
- FBS:
-
Fetal bovine serum
- FFA:
-
Free fatty acids
- HEPES:
-
Phenylethylamine and 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- LCPUFA:
-
Long chain polyunsaturated fatty acids
- LDCF 2, 7:
-
Dichlorofluorescein
- MG:
-
Monoglycerides
- MUFA:
-
Monounsaturated fatty acids
- PBS:
-
Phosphate buffer saline
- PCR:
-
Polymerase chain reaction
- PL:
-
Phospholipids
- PPAR:
-
Peroxisome proliferator-activated receptors
- PUFA:
-
Polyunsaturated fatty acid
- RPL2 RNA:
-
Polymerase II polypeptide
- SAFA:
-
Saturated fatty acids
- SRB-I:
-
Scavenger receptor type B
- SREBP-1:
-
Sterol regulatory element-binding protein-1
- TAG:
-
Triacylglycerols
- TLC:
-
Thin-layer chromatography
- VLDL:
-
Very low-density lipoprotein
- Δ5:
-
Δ5 Desaturase
- Δ6:
-
Δ6 Desaturase
References
Tacon AGJ (2008) State of information on salmon aquaculture feed and the environment. Report prepared for the WWF US Initiated Salmon Aquaculture Dialogue, p 80, http://www.westcoastaquatic.ca/Aquaculture_feed_environment.pdf(accessed March 2008)
FAO (2007) Faostat, http://www.faostat.fao.org/ (accessed November 2007)
Torstensen BE, Bell JG, Rosenlund G, Henderson RJ, Graff IE, Tocher DR, Lie O, Sargent JR (2005) Tailoring of Atlantic salmon (Salmo salar L.) flesh lipid composition and sensory quality by replacing fish oil with a vegetable oil blend. J Agri Food Chem 53:10166–10178
Ackman RG (1996) DHA: can it benefit salmon marketing? J Aquatic Food Prod Tech 5:7–26
Trattner S, Kamal-Eldin A, Brännäs E, Moazzami A, Zlabek V, Larsson P, Ruyter B, Gjøen T, Pickova J (2008) Sesamin supplementation increases white muscle docosahexaenoic acid (DHA) levels in rainbow trout (Oncorhynchus mykiss) fed high alpha-linolenic acid (ALA) containing vegetable oil: metabolic actions. Lipids. doi:10.1007/s11745-008-3228-8
Kiso Y, Tsuruoka N, Kidokoro A, Matsumoto I, Abe K (2005) Sesamin ingestion regulates the transcription levels of hepatic metabolizing enzymes for alcohol and lipids in rats. Alcohol Clin Exp Res 29:116S–120S
Jeng KCG, Hou RCW (2005) Sesamin and sesamolin: nature’s therapeutic lignans. Curr Enzym Inhib 1:11–20
Ashakumary L, Rouyer I, Takahashi Y, Ide T, Fukuda N, Aoyama T, Hashimoto T, Mizugaki M, Sugano M (1999) Sesamin, a sesame lignan, is a potent inducer of hepatic fatty acid oxidation in the rat. Metabolism 48:1303–1313
Fujiyama-Fujiwara Y, Umeda-Sawada R, Kuzuyama M, Igarashi O (1995) Effects of sesamin on the fatty acid composition of the liver of rats fed n-6 and n-3 fatty acids-rich diet. J Nutr Sci Vitaminol 41:217–225
Kushiro M, Masaoka T, Hageshita S, Takahashi Y, Ide T, Sugano M (2002) Comparative effect of sesamin and episesamin on the activity and gene expression of enzymes in fatty acid oxidation and synthesis in rat liver. J Nutri Biochem 13:289–295
Kamal-Eldin A, Frank J, Razdan A, Tengblad S, Basu S, Vessby B (2000) Effects of dietary phenolic compounds on tocopherol, cholesterol, and fatty acids in rats. Lipids 35:427–435
Ide T, Hong DD, Ranasinghe P, Takahashi Y, Kushiro M, Sugano M (2004) Interaction of dietary fat types and sesamin on hepatic fatty acid oxidation in rats. Biochim Biophys Acta 1682:80–91
Seglen PO (1976) Preparation of isolated rat liver cells. Methods Cell Biol 13:29–83
Dannevig BH, Berge T (1985) Endocytosis of galactose-terminated glycoproteins by isolated liver cells of the rainbow trout (Salmo gairdneri). Comp Biochem Physiol B 82:683–688
Kjaer MA, Vegusdal A, Gjoen T, Rustan AC, Todorcevic M, Ruyter B (2008) Effect of rapeseed oil and dietary n-3 fatty acids on triacylglycerol synthesis and secretion in Atlantic salmon hepatocytes. Biochim Biophys Acta 1781:112–122
Folch J, Lees M, Stanley SG H (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Narce M, Gresti J, Bezard J (1988) Method for evaluating the bioconversion of radioactive polyunsaturated fatty acids by use of reversed-phase liquid chromatography. J Chromatogr A 448:249–264
Hara A, Radin NS (1978) Lipid extraction of tissue with low toxicity solvent. Anal Biochem 90:420–426
Appelqvist L (1968) Rapid methods of lipid extractions and fatty acid methyl ester preparation for seed and leaf tissue with special remarks on preventing the accumulation of lipids contaminants. R Swedish Acad of Sci 28:551–570
Fredriksson S, Elwinger K, Pickova J (2006) Fatty acid and carotenoid composition of egg yolk as an effect of microalgae addition to feed formula for laying hens. Food Chem 99:530–537
Christiansen R, Borrebaek B, Bremer J (1976) The effect of (-)carnitine on the metabolism of palmitate in liver cells isolated from fasted and refed rats. FEBS Lett 62:313–317
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Peterson GL (1977) A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem 83:346–356
Small GM, Burdett K, Connock MJ (1985) A sensitive spectrophotometric assay for peroxisomal acyl-CoA oxidase. Biochem J 227:205–210
Leighton F, Bergseth S, Rortveit T, Christiansen EN, Bremerll J (1989) Free acetate production by rat hepatocytes during peroxisomal fatty acid and dicarboxylic acid oxidation. J Biol Chem 264:10347–10350
Voss A, Reinhart M, Sankarappa S, Sprecher H (1991) The metabolism of 7, 10, 13, 16, 19-docosapentaenoic acid to 4, 7, 10, 13, 16, 19-docosahexaenoic acid in rat-liver is independent of a Δ4-Desaturase. J Biol Chem 266:19995–20000
Kiessling KH, Kiessling A (1993) Selective utilization of fatty-acids in rainbow-trout (Oncorhynchus-Mykiss Walbaum) red muscle mitochondria. Can J Zool 71:248–251
Umeda-Sawada R, Ogawa M, Igarashi O (1998) The metabolism and n-6/n-3 ratio of essential fatty acids in rats: effect of dietary arachidonic acid and mixture of sesame lignans (sesamin and episesamin). Lipids 33:567–572
Vegusdal A, Ostbye TK, Tran TN, Gjoen T, Ruyter B (2004) Beta-oxidation, esterification, and secretion of radiolabeled fatty acids in cultivated Atlantic salmon skeletal muscle cells. Lipids 39:649–658
Ferre P (2004) The biology of peroxisome proliferator—activated receptors—relationship with lipid metabolism and insulin sensitivity. Diabetes 53:S43–S50
Ruyter B, Andersen O, Dehli A, Farrants AKO, Gjoen T, Thomassen MS (1997) Peroxisome proliferator activated receptors in Atlantic salmon (Salmo salar): effects on PPAR transcription and acyl-CoA oxidase activity in hepatocytes by peroxisome proliferators and fatty acids. Biochim Biophys Acta 1348:331–338
Andersen Ø, Eijsink VGH, Thomassen M (2000) Multiple variants of the peroxisome proliferator-activated receptor (PPAR) [gamma] are expressed in the liver of Atlantic salmon (Salmo salar). Gene 255:411–418
Todorčević M, Vegusdal A, Gjøen T, Sundvold H, Torstensen EB, Kjær AM, Ruyter B (2008) Changes in fatty acids metabolism during differentiation of Atlantic salmon preadipocytes; Effects of n-3 and n-9 fatty acids. Biochim Biophys Acta 1781:326–335
Lee CH, Olson P, Evans RM (2003) Minireview: lipid metabolism, metabolic diseases, and peroxisome proliferator-activated receptors. Endocrinology 144:2201–2207
Acknowledgments
This study was financed by the Swedish research councils, Vetenskapsrådet and FORMAS, and the European Union Structural Funding for Fisheries. The authors like to thank Inger Ø Kristiansen at A.S. Nofima for valuable and skillful technical assistance.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Trattner, S., Ruyter, B., Østbye, T.K. et al. Sesamin Increases Alpha-Linolenic Acid Conversion to Docosahexaenoic Acid in Atlantic Salmon (Salmo salar L.) Hepatocytes: Role of Altered Gene Expression. Lipids 43, 999–1008 (2008). https://doi.org/10.1007/s11745-008-3229-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-008-3229-7