Abstract
Surface-active properties of cleavable surfactants with a betaine ester group—(n-alkyloxycarbonylmethyl)trimethylammonium chlorides, used as separation reagents—were investigated. Critical micelle concentrations, dispersing powers, and foaming powers were comparable to those of alkyltrimethylammonium chlorides with the same total number of carbon atoms. On the other hand, the solubilities of the four hydrophobic dyes N,N-dimethyl-3-nitroaniline, naphthalene, pyrene, and oil orange SS in the former surfactant solutions were equal to or slightly smaller than those in the solutions of the latter surfactants with the same alkyl chain length. The alkali hydrolysis yields of the formers approached 100% in aqueous buffer solution at pH 10 and 25 °C within 10 min, and the yield at pH 9 was dependent on the alkyl chain length. This type of surfactant was also found to be as an efficient separation reagent which disperses carbon black and solubilizes the above dyes into aqueous neutral solution and then separates them instantaneously and almost perfectly as precipitates when a small excess of NaOH is added.






Similar content being viewed by others
Abbreviations
- CMC:
-
Critical micelle concentration
- C n B:
-
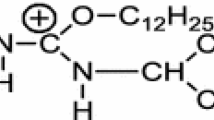
(n-Alkyloxycarbonylmethyl)trimethylammonium chloride
- C n TAC:
-
n-Alkyltrimethylammonium chlorides
- DMNA:
-
N,N-Dimethyl-3-nitroaniline
- PyCHO:
-
1-Pyrenecarboxyaldehyde
- [dye]s (M):
-
concentration of dye solubilized in surfactant solution at a given surfactant concentration
- [dye]CMC (M):
-
concentration of dye solubilized in surfactant solution at CMC
- ε :
-
dielectric constant
- λ max (nm):
-
fluorescence maximum wavelength
- n :
-
number of carbon atoms of a sequential alkyl chain
- S :
-
solubilizing power
- [surfactant]t (M):
-
total surfactant concentration
References
Jaeger DA (1995) Cleavable surfactants. Supramol Chem 5:27–30. doi:10.1080/10610279508029884
Stjerndahl M, Lundberg D, Holmberg K (2003) Cleavable surfactants. In: Holmberg K (ed) Novel surfactants: preparation, applications, and biodegradability (Surfactant Science Series 114). CRC, London, pp 317–345
Mohlin K, Karlsson P, Holmberg K (2006) Use of cleavable surfactants for alkyl ketene dimer (AKD) dispersions. Colloids Surf A Physicochem Eng Asp 274:200–210. doi:10.1016/j.colsurfa.2005.07.038
Iyer M, Hayes DG, Harris JM (2001) Synthesis of pH-degradable nonionic surfactants and their applications in microemulsions. Langmuir 17:6816–6821. doi:10.1021/la010494tS0743-7463(01)00494-2
Rairkar ME, Hayes DG, Harris JM (2007) Solubilization of enzymes in water-in-oil microemulsions and their rapid and efficient release through use of a pH-degradable surfactant. Biotechnol Lett 29:767–771. doi:10.1007/s10529-006-9292-3
Guo X, MacKay JA, Szoka FC Jr (2003) Mechanism of pH-triggered collapse of phosphatidylethanolamine liposomes stabilized by an ortho ester polyethyleneglycol lipid. Biophys J 84:1784–1795
Zhu J, Munn RJ, Nantz MH (2000) Self-cleaving ortho ester lipids: a new class of pH-vulnerable amphiphiles. J Am Chem Soc 122:2645–2646. doi:10.1021/ja994149q
By K, Nantz MH (2004) Dioxazocinium ortho esters: a class of highly pH-vulnerable amphiphiles. Angew Chem Int Ed Engl 116:1137–1140. doi:10.1002/ange.200352589
Itoh Y, Takahashi K, Uemura Y (2008) Solvent extraction of DNA with a hydrolysable double-chain surfactant. Sep Sci Tech (in press)
Itoh Y, Akasaka R, Takahashi K (2008) Preparation of emulsifier-free polystyrene by conventional emulsion polymerization with a hydrolysable emulsifier. J Appl Polym Sci 108:358–361. doi:10.1002/app.27654
Itoh Y, Akasaka R, Sahara F (2008) Facile polymer coating of papers with polymer latices containing a hydrolysable emulsifier. J Appl Polym Sci (in press)
Lindstedt M, Allenmark S, Thompson RA, Edebo L (1990) Antimicrobial activity of betaine esters, quaternary ammonium amphiphiles which spontaneously hydrolyze into nontoxic components. Antimicrob Agents Chemother 34:1949–1954
Thompson RA, Allenmark S (1992) Factors influencing the micellar catalyzed hydrolysis of long-chain alkyl betainates. J Colloid Interface Sci 148:241–246. doi:10.1016/0021-9797(92)90132-6
Lundberg D, Holmberg K (2004) Nuclear magnetic resonance studies on hydrolysis kinetics and micellar growth in solutions of surface-active betaine esters. J Surfactants Deterg 7:239–246. doi:10.1007/s11743-004-0307-9
Menger FM, Galloway AL (2004) Contiguous versus segmented hydrophobicity in micellar systems. J Am Chem Soc 126:15883–15889. doi:10.1021/ja040105s
Itoh Y, Horiuchi J, Takahashi K (2007) Alkylaldehyde-bisulfite adducts as cleavable surfactants. Colloids Surf A Physicochem Eng Asp 308:118–122. doi:10.1016/j.colsurfa.2007.05.037
Kalyanasundaram K, Thomas JK (1977) Solvent-dependent fluorescence of pyrene-3-carboxyaldehyde and its applications in the estimation of polarity at micelle–water interfaces. J Phys Chem 81:2176–2180. doi:10.1021/j100538a008
Rozycka-Roszak B, Przestalski S, Witek S (1988) Calorimetric studies of the micellization of some amphiphilic betaine ester derivatives. J Colloid Interface Sci 125:80–85. doi:10.1016/0021-9797(88)90056-2
Mukerjee P, Mysels KJ (1971) Critical micelle concentrations of aqueous surfactant systems (National Standards Reference Data Series, vol 36). National Bureau of Standards, Washington, DC
Ridaoui H, Jada A, Vidal L, Donnet J-B (2006) Effect of cationic surfactant and block copolymer on carbon black particle surface charge and size. Colloids Surf A Physicochem Eng Asp 278:149–159. doi:10.1016/j.colsurfa.2005.12.013
Parida SK, Dash S, Patel S, Mishra BK (2006) Adsorption of organic molecules on silica surface. Adv Colloid Interface Sci 121:77–110. doi:10.1016/j.cis.2006.05.028
Namba Y, Hayashi S, Fuchizawa T (1955) Studies on the factors affecting detergency. I. About sodium soaps of saturated fatty acids. Yukagaku 4:238–244
Hayashi S, Yamamoto T, Fuchizawa T, Namba Y (1957) Studies on the factors affecting detergency. III. About sodium salts of saturated fatty alcohol sulfates. Yukagaku 6:213–217
Takada M, Kurihara M, Shibuya S, Kitamura K, Matsumoto A, Moriya M, Hidaka H (1996) Fundamental properties of amphoteric surfactant 3-(N′-acyl)-aminopropyl-N,N-dimethyl-N-carboxymethyl betaine. J Jpn Oil Chem Soc 45:1239–1246
Porter MR (1994) Chapter 4. In: Handbook of surfactants, 2nd edn. Blackie, Glasgow
Edwards DA, Luthy RG, Liu Z (1991) Solubilization of polycyclic aromatic hydrocarbons in micellar nonionic surfactant solutions. Environ Sci Technol 25:127–133. doi:10.1021/es00013a014
Sangster Research Laboratories (2008) LOGKOW—a databank of evaluated octanol–water partition coefficients (log P). http://logkow.cisti.nrc.ca/logkow/, accessed on August 2008
Virtual Computational Chemistry Laboratory (2008) ALOGPS 2.1. http://www.vcclab.org/lab/alogps/, accessed on August 2008
Abe A, Imae T, Ikeda S (1987) Solubilization properties of aqueous solutions of alkyltrimethylammonium halides toward a water-insoluble dye. Colloid Polym Sci 265:637–645. doi:10.1007/BF01412780
Christian SD, Scamehorn JF (1995) Solubilization in surfactant aggregates (Surfactant Science Series 55). CRC, London
Turro NJ, Okubo T (1982) Polarity at the micelle–water interface under high pressure as estimated by a pyrene-3-carboxyaldehyde fluorescence probe. J Phys Chem 86:159–161. doi:10.1021/j100391a003
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research (No. 20550168) and a Grant-in-Aid for Global COE program from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Itoh, Y., Akasaka, R. Comparison of Surface-Active Properties of (Alkyloxycarbonylmethyl)trimethylammonium Chlorides and Alkyltrimethylammonium Chlorides. J Surfact Deterg 12, 101–107 (2009). https://doi.org/10.1007/s11743-008-1097-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-008-1097-z