Abstract
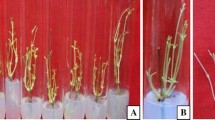
Since a decade, the large-scale commercial production of Siratia grosvenorii plantlets is being practiced through in vitro culture of its microcuttings, but it has some drawbacks such as handling of plantlets, low transplant-survival rate, development of massive callus, low yield after transplantation, etc. An experiment has been conducted to improve the prevailing technique as well as to develop a new ex vitro technique to overcome these drawbacks. Several concentrations of naphthalene acetic acid (NAA) (0–4.0 mg/l) have been tried with the MS (Murashige and Skoog in Physiol Plant 15:473–479, 1962) basal medium containing 3% (w/v) sucrose and 4.0 g/l agar, out of which 0.1 mg/l NAA was found best in terms of smaller diameter of callus and maximum rooting and transplant survival rate. Further, use of perlite instead of agar medium also showed possibilities for future research on commercial-scale plantlet production. Ex vitro rooting technique was found superior to the in vitro one as plantlets developed through this method had lateral roots without any callus at the base of microcuttings, just like the natural root system and of course with higher root length, rooting rates, and transplant survival rate compared to the in vitro developed plantlets. Further, this technique is economical in terms of labor and time saving and gives rise to vigorous plants which ultimately bring higher yields and profits.


Similar content being viewed by others
References
Afreen F, Zobayed SMA, Kubota C, Kozai T, Hasegawa O (1999) Supporting material affects the growth and development of in vitro sweet potato plantlets cultured photoautotrophically. In Vitro Cell Dev Biol Plant 35:470–474
Ahmad N, Anis M (2005) In vitro mass propagation of Cucumis sativus L. from nodal segments. Turk J Bot 29:237–240
Andersen AS (1986) Environmental influences on adventitious rooting in cuttings of non-woody species. In: Jackson MB (ed) New root formation in plants and cuttings. Martinus Nijhoff Publishers, Netherlands, pp 223–253
Bhatia NP, Bhatia P, Ashwath N (2002) Ex vitro rooting of micropropagated shoots of Stackhousia tryonii. Biol Plant 45:441–444
Bozena B (2001) Morphological and physiological characteristics of micropropagated strawberry plants rooted in vitro or ex vitro. Sci Hort 89:195–206
Debergh PC, Maene LJA (1981) A scheme for commercial propagation of ornamental plants by tissue culture. Sci Hort 14:335–345
Feyissa T, Weiander M, Negash L (2007) Genetic stability, ex vitro rooting and gene expression studies in Hagenia abyssinica. Biol Plant 51:15–21
Geert-Jan DK, Jolanda TB, Svetla M (1997) Effectiveness of indoleacetic acid, indolebutyric acid and naphthaleneacetic acid during adventitious root formation in vitro in Malus “Jork 9”. Plant Cell Tissue Organ Cult 49:39–44
Karhu ST (1997) Rooting of blue honeysuckle microshoots. Plant Cell Tissue Organ Cult 48:153–159
Kataoka I (1994) Influence of rooting substrates on the morphology of papaya root formed in vitro. Jpn J Trop Agr 38:251–257
Kim MS, Klopfenstein NB, Cregg BM (1998) In vitro and ex vitro rooting of micropropagated shoots using three green ash (Fraxinus pennsylvanica) clones. New Forests 16:43–57
Kirdmanee C, Kitaya Y, Kozai T (1995) Effects of CO2 enrichment and supporting material in vitro on photoautotrophic growth of Eucalyptus plantlets in vitro and ex vitro: anatomical comparisons. Acta Hortic 393:111–115
Kozai T, Afreen F, Zobayed SMA (2005) Photoautotrophic (sugar-free medium) micropropagation as a new micropropagation and transplant production system. Springer, Dordrecht
Lin R, Wang RZ (1980) The whole plantlets obtained of S. grosvenorii via plant tissue culture. Guihaia 1:11 [In Chinese]
Lin W, Li QQ, Peng HW, Xue JJ, Liang S, Huang LY (2003) Problem and solution of tissue cultured seedling cultivation of S. grosvenorii. Guangxi Agr Sci 4:74–75 [In Chinese]
Lu AM, Zhang ZY (1984) The genus S. Merr. in China. Guihaia 4:27–33 [In Chinese]
Martin KP (2003) Rapid in vitro multiplication and ex vitro rooting of Rotula aquatica Lour., a rare rhoeophytic woody medicinal plant. Plant Cell Rep 21:415–420
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–479
Preece JE, Sutter EG (1991) Acclimatization of micropropagated plants to the greenhouse and field. In: Debergh PC, Zimmerman RH (eds) Micropropagation technology and application. Kluwer, Dordrecht, pp 71–93
Pruski KW, Lewis T, Astatkie T, Nowak J (2000) Micropropagation of Chokecherry and Pincherry cultivars. Plant Cell Tissue Organ Cult 63:93–100
Saini R, Jaiwal PK (2000) In vitro multiplication of Peganum harmala—an important medicinal plant. Indian J Exp Biol 38:499–503
Sanchez MC, San-Jose MC, Ballester A, Vieitez AM (1996) Requirements for in vitro rooting of Quercus robur and Q. rubra shoots derived from mature trees. Tree Physiol 16:673–680
Sha Valli Khan PS, Kozai T, Nguyen QT, Kubota C, Dhawan V (2002) Growth and net photosynthetic rates of Eucalyptus tereticornis Smith under photomixotrophic and various photoautotrophic micropropagation conditions. Plant Cell Tissue Organ Cult 71:141–146
Xu J, Wang YZ, Zhang YX, Chai TY (2008) Rapid in vitro multiplication and ex vitro rooting of Malus zumi (Matsumura) Rehd. Acta Physiol Plant 30:129–132
Acknowledgments
This research was financially supported by Innovation Foundation for Ph.D. of Guangxi University, and Research Foundation granted by Guangxi Academy of Agricultural Sciences, Nanning, Guangxi, PR China. We are also grateful to Dr. Manoj K. Srivastava and Dr. Dalvi Vijay from India for their kind help in manuscript revision.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by E. Lojkowska.
Rights and permissions
About this article
Cite this article
Yan, H., Liang, C., Yang, L. et al. In vitro and ex vitro rooting of Siratia grosvenorii, a traditional medicinal plant. Acta Physiol Plant 32, 115–120 (2010). https://doi.org/10.1007/s11738-009-0386-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-009-0386-0