Abstract
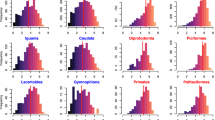
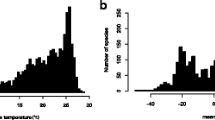
Species geographical ranges are at the core of many areas in evolutionary biology, yet empirical studies on the evolution of geographical ranges have been limited. Here, we integrate information on the phylogenetic relationships and geographical distribution of 3097 species of mammal (Artiodactyla, Carnivora, Chiroptera, Marsupialia, and Primates) and squamate (Anguimorpha, Gekkota, Iguania, Lacertoidea, Scincoidea, and Serpentes) to assess the degree of evolutionary “heritability” (i.e., phylogenetic autocorrelation) in range sizes and the extent to which range limits at higher and lower latitudes share similar evolutionary rates. Phylogenetic autocorrelation was highly variable among clades in the case of range size, but invariably high for range latitudinal centroid and range limits. Moreover, rates of evolution of high-latitude limits were 1.6–4 times faster than low-latitude limits. These results are consistent with previous experimental studies showing that heat tolerance is conserved across lineages, whereas tolerance to cold temperatures is more labile. The distinct evolutionary rates of low- and high-latitude limits has important implications for our understanding of the evolution of geographical ranges, as well as to understand how they could be affected by predicted anthropogenic climate changes.




Similar content being viewed by others
References
Alfaro, M. E., Santini, F., Brock, C., Alamillo, H., Dornburg, A., Rabosky, D. L., et al. (2009). Nine exceptional radiations plus high turnover explain species diversity in jawed vertebrates. Proceedings of the National Academy of Sciences, 106(32), 13410–13414. doi:10.1073/pnas.0811087106.
Araújo, M. B., Ferri-Yáñez, F., Bozinovic, F., Marquet, P. A., Valladares, F., & Chown, S. L. (2013). Heat freezes niche evolution. Ecology Letters, 16(9), 1206–1219. doi:10.1111/ele.12155.
Bininda-Emonds, O. R. P., Cardillo, M., Jones, K. E., MacPhee, R. D. E., Beck, R. M. D., Grenyer, R., et al. (2007). The delayed rise of present-day mammals. Nature, 446(7135), 507–512. doi:10.1038/nature05634.
Blackburn, T. M., & Gaston, K. J. (1996). Spatial patterns in the geographic range sizes of bird species in the new world. Philosophical Transactions of the Royal Society B: Biological Sciences, 351(1342), 897–912. doi:10.1098/rstb.1996.0083.
Blomberg, S. P., Garland, T., & Ives, A. R. (2003). Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution, 57(4), 717–745. doi:10.1111/j.0014-3820.2003.tb00285.x.
Borregaard, M. K., Gotelli, N. J., & Rahbek, C. (2012). Are range-size distributions consistent with species-level heritability? Evolution, 66(7), 2216–2226. doi:10.1111/j.1558-5646.2012.01581.x.
Cahill, A. E., Aiello-Lammens, M. E., Caitlin Fisher-Reid, M., Hua, X., Karanewsky, C. J., Ryu, H. Y., et al. (2014). Causes of warm-edge range limits: Systematic review, proximate factors and implications for climate change. Journal of Biogeography, 41(3), 429–442. doi:10.1111/jbi.12231.
Cardillo, M. (2015). Geographic range shifts do not erase the historic signal of speciation in mammals. The American Naturalist, 185(3), 343–353. doi:10.1086/679663.
Carotenuto, F., Barbera, C., & Raia, P. (2010). Occupancy, range size, and phylogeny in Eurasian Pliocene to recent large mammals. Paleobiology, 36(3), 399–414. doi:10.1666/09059.1.
Clavel, J., Escarguel, G., & Merceron, G. (2015). mvMORPH: An R package for fitting multivariate evolutionary models to morphometric data. Methods in Ecology and Evolution, 6(11), 1311–1319. doi:10.1111/2041-210x.12420.
Cunningham, H. R., Rissler, L. J., Buckley, L. B., & Urban, M. C. (2016). Abiotic and biotic constraints across reptile and amphibian ranges. Ecography, 39(1), 1–8. doi:10.1111/ecog.01369.
Diniz-Filho, J. A. F. (2004). Macroecology and the hierarchical expansion of evolutionary theory. Global Ecology and Biogeography, 13(1), 1–5. doi:10.1111/j.1466-882x.2004.00066.x.
FitzJohn, R. G. (2012). Diversitree: Comparative phylogenetic analyses of diversification in R. Methods in Ecology and Evolution, 3(6), 1084–1092. doi:10.1111/j.2041-210x.2012.00234.x.
Freckleton, R. P., Harvey, P. H., & Pagel, M. (2002). Phylogenetic analysis and comparative data: A test and review of evidence. The American Naturalist, 160(6), 712–726. doi:10.1086/343873.
Freckleton, R. P., & Jetz, W. (2009). Space versus phylogeny: Disentangling phylogenetic and spatial signals in comparative data. Proceedings of the Royal Society B: Biological Sciences, 276(1654), 21–30. doi:10.1098/rspb.2008.0905.
Fritz, S. A., Bininda-Emonds, O. R. P., & Purvis, A. (2009). Geographical variation in predictors of mammalian extinction risk: big is bad, but only in the tropics.” Ecology Letters, 12(6), 538–549. doi:10.1111/j.1461-0248.2009.01307.x.
Gaston, K. J. (1998). Species-range size distributions: Products of speciation, extinction and transformation. Philosophical Transactions of the Royal Society B: Biological Sciences, 353(1366), 219–230. doi:10.1098/rstb.1998.0204.
Gaston, K. J., & He, F. (2002). The distribution of species range size: A stochastic process. Proceedings of the Royal Society B: Biological Sciences, 269(1495), 1079–1086. doi:10.1098/rspb.2002.1969.
Gittleman, J. L., & Kot, M. (1990). Adaptation: Statistics and a null model for estimating phylogenetic effects. Systematic Biology, 39(3), 227. doi:10.2307/2992183.
Grantham, T. A. (1995). Hierarchical approaches to Macroevolution: Recent work on species selection and the “effect hypothesis.” Annual Review of Ecology and Systematics, 26, 301–321. doi:10.2307/2097209.
Harmon, L. J., Weir, J. T., Brock, C. D., Glor, R. E., & Challenger, W. (2008). GEIGER: Investigating evolutionary radiations. Bioinformatics (Oxford, England), 24(1), 129–131. doi:10.1093/bioinformatics/btm538.
Hunt, G., Roy, K., & Jablonski, D. (2005). Species-level heritability reaffirmed: A comment on “On the heritability of geographic range Sizes.” The American Naturalist, 166(1), 129–135. doi:10.1086/430722.
IUCN. (2013). The IUCN Red List of Threatened Species. http://www.iucnredlist.org.
Jablonski, D. (1987). Heritability at the species level: Analysis of geographic ranges of Cretaceous mollusks. Science, 238(4825), 360–363. doi:10.1126/science.238.4825.360.
Jetz, W., Thomas, G. H., Joy, J. B., Hartmann, K., & Mooers, A. O. (2012). The global diversity of birds in space and time. Nature, 491(7424), 444–448. doi:10.1038/nature11631.
Jones, K. E., Sechrest, W., & Gittleman, J. L. (2005). Age and area revisited: identifying global patterns and implications for conservation. In A. Purvis, J. L. Gittleman & T. M. Brooks (Eds.), Phylogeny and conservation (pp. 141–165). Cambridge: Cambridge University Press.
Komsta, L., & Novomestky, F. (2015). moments: Moments, cumulants, skewness, kurtosis and related tests. R package version 0.14. http://CRAN.R-project.org/package=moments.
Lawson, A. M., & Weir, J. T. (2014). Latitudinal gradients in climatic-niche evolution accelerate trait evolution at high latitudes. Ecology Letters, 17(11), 1427–1436. doi:10.1111/ele.12346.
Lewontin, R. C. (1970). The units of selection. Annual Review of Ecology and Systematics, 1, 1–18. doi:10.2307/2096764.
Louthan, A. M., Doak, D. F., & Angert, A. L. (2015). Where and when do species interactions set range limits? Trends in Ecology & Evolution, 30(12), 780–792. doi:10.1016/j.tree.2015.09.011.
MacArthur, R. H. (1972). Geographical ecology: Patterns in the distribution of species. New York: Harper & Row.
Machac, A., Zrzavý, J., & Storch, D. (2011). Range size heritability in Carnivora is driven by geographic constraints. The American Naturalist, 177(6), 767–779. doi:10.1086/659952.
Meredith, R. W., Janecka, J. E., Gatesy, J., Ryder, O. A., Fisher, C. A., Teeling, E. C., et al. (2011). Impacts of the Cretaceous terrestrial revolution and KPg extinction on mammal diversification. Science, 334(6055), 521–524. doi:10.1126/science.1211028.
Mittelbach, G. G., Schemske, D. W., Cornell, H. V., Allen, A. P., Brown, J. M., Bush, M. B., et al. (2007). Evolution and the latitudinal diversity gradient: Speciation, extinction and biogeography. Ecology Letters, 10(4), 315–331. doi:10.1111/j.1461-0248.2007.01020.x.
Mouillot, D., & Gaston, K. (2009). Spatial overlap enhances geographic range size conservatism. Ecography, 32(4), 671–675. doi:10.1111/j.1600-0587.2009.05679.x.
Munkemuller, T., Lavergne, S., Bzeznik, B., Dray, S., Jombart, T., Schiffers, K., Thuiller, T. (2012). How to measure and test phylogenetic signal. Methods Ecol. Evol, 3, 743–756.
Olalla-Tárraga, M. Á., McInnes, L., Bini, L. M., Diniz-Filho, J. A. F., Fritz, S. A., Hawkins, B. A., et al. (2011). Climatic niche conservatism and the evolutionary dynamics in species range boundaries: Global congruence across mammals and amphibians. Journal of Biogeography, 38(12), 2237–2247. doi:10.1111/j.1365-2699.2011.02570.x.
Pagel, M. (1999). Inferring the historical patterns of biological evolution. Nature, 401(6756), 877–884. doi:10.1038/44766.
Paradis, E., Claude, J., & Strimmer, K. (2004). APE: Analyses of phylogenetics and evolution in R language. Bioinformatics (Oxford, England), 20(2), 289–290. doi:10.1093/bioinformatics/btg412.
Pianka, E. R. (1970). Comparative autecology of the lizard Cnemidophorus tigris in different parts of its georgraphic range. Ecology, 51(4), 703–720. doi:10.2307/1934053.
Pigot, A. L., Phillimore, A. B., Owens, I. P. F., & Orme, C. D. L. (2010). The shape and temporal dynamics of phylogenetic trees arising from geographic speciation. Systematic Biology, 59(6), 660–673. doi:10.1093/sysbio/syq058.
Pigot, A. L., & Tobias, J. A. (2015). Dispersal and the transition to sympatry in vertebrates. Proceedings of the Royal Society B: Biological Sciences, 282(1799), 20141929–20141929. doi:10.1098/rspb.2014.1929.
Pither, J. (2003). Climate tolerance and interspecific variation in geographic range size. Proceedings of the Royal Society B: Biological Sciences, 270(1514), 475–481. doi:10.1098/rspb.2002.2275.
Pyron, M. (1999). Relationships between geographical range size, body size, local abundance, and habitat breadth in North American suckers and sunfishes. Journal of Biogeography, 26(3), 549–558. doi:10.1046/j.1365-2699.1999.00303.x.
Pyron, R., Burbrink, F. T., & Wiens, J. J. (2013). A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMC Evolutionary Biology, 13(1), 93. doi:10.1186/1471-2148-13-93.
Pyron, R. A., & Burbrink, F. T. (2014). Early origin of viviparity and multiple reversions to oviparity in squamate reptiles. Ecology Letters, 17(1), 13–21. doi:10.1111/ele.12168.
R Core Team. (2015). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. http://www.R-project.org.
Rabosky, D. L., & Goldberg, E. E. (2015). Model inadequacy and mistaken inferences of trait-dependent speciation. Systematic Biology, 64(2), 340–355. doi:10.1093/sysbio/syu131.
Rabosky, D. L., Grundler, M., Anderson, C., Title, P., Shi, J. J., Brown, J. W., et al. (2014). BAMMtools: An R package for the analysis of evolutionary dynamics on phylogenetic trees. Methods in Ecology and Evolution, 5(7), 701–707. doi:10.1111/2041-210x.12199.
Revell, L. J. (2012). phytools: An R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution, 3(2), 217–223. doi:10.1111/j.2041-210x.2011.00169.x.
Ricklefs, R. E. (1987). Community diversity: Relative roles of local and regional processes. Science, 235(4785), 167–171. doi:10.1126/science.235.4785.167.
Ricklefs, R. E., & Latham, R. E. (1992). Intercontinental correlation of geographical ranges suggests stasis in ecological traits of relict genera of temperate perennial herbs. The American Naturalist, 139(6), 1305–1321. doi:10.2307/2462343.
Saupe, E. E., Qiao, H., Hendricks, J. R., Portell, R. W., Hunter, S. J., Soberón, J., & Lieberman, B. S. (2015). Niche breadth and geographic range size as determinants of species survival on geological time scales. Global Ecology and Biogeography, 24(10), 1159–1169. doi:10.1111/geb.12333.
Stanley, S. M. (1975). A theory of evolution above the species level. Proceedings of the National Academy of Sciences, 72(2), 646–650. Retrieved March 1, 2016 from http://www.pnas.org/content/72/2/646.short.
Villalobos, F., Rangel, T. F., & Diniz-Filho, J. A. F. (2013). Phylogenetic fields of species: Cross-species patterns of phylogenetic structure and geographical coexistence. Proceedings of the Royal Society B: Biological Sciences, 280(1756), 20122570–20122570. doi:10.1098/rspb.2012.2570.
Wagner, P. J., & Erwin, D. H. (1995). Phylogenetic patterns as tests of speciation hypotheses. In D. H. Erwin & R. L. Anstey (Eds.), New approaches to speciation in the fossil record (pp. 87–122). New York: Columbia University Press.
Waldron, A. (2007). Null models of geographic range size evolution reaffirm its Heritability. The American Naturalist, 170(2), 221–231. doi:10.1086/518963.
Webb, T. J., & Gaston, K. J. (2003). On the heritability of geographic range sizes. The American Naturalist, 161(4), 553–566. doi:10.1086/368296.
Webb, T. J., & Gaston, K. J. (2005). Heritability of geographic range sizes revisited: A reply to Hunt et al. The American Naturalist, 166(1), 136–143. doi:10.1086/430726.
Acknowledgements
We thank A. Duran, Michael K. Borregaard and an anonymous reviewer for valuable comments on an earlier version of this manuscript. MRP was supported by a CNPq/MCT grant (571334/2008-3). ALSM is supported by doctoral fellowship from CAPES.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pie, M.R., Meyer, A.L.S. The Evolution of Range Sizes in Mammals and Squamates: Heritability and Differential Evolutionary Rates for Low- and High-Latitude Limits. Evol Biol 44, 347–355 (2017). https://doi.org/10.1007/s11692-017-9412-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11692-017-9412-0