Abstract
Background
Microsporidia infection was originally described as an immunocompromised associated pathogen. Limitations to correct microscopic diagnosis of microsporidia include size of the organism presenting a challenge even to a highly competent laboratory expert.
Objective
The present study aimed to detect microsporidia infection among leukemic children. The performance of modified trichrome stain and PCR in the diagnosis of microsporidia was evaluated with further speciation.
Methods
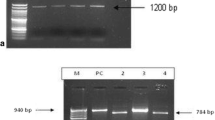
Stool samples of 100 leukemic children on chemotherapy were examined microscopically for microsporidia. DNA was extracted from all samples. Amplification was performed by conventional and nested PCR. Sequencing of amplified products was performed on unspeciated samples.
Results
Microsporidia were detected in 23% of the children by MTS and 29% by PCR. The 29 positive samples were subjected to PCR for speciation. Enterocytozoon bieneusi was found to predominate in 20 cases, Encephalitozoon intestinalis in three cases, two cases had co-infection, and the remaining four samples were not amplified with either E. bieneusi or E. intestinalis specific primers. By DNA sequencing of the unspeciated samples, three samples shared high homology with Encephalitozoon hellem and one sample with Encephalitozoon cuniculi. Referring to PCR as a gold standard, MTS exhibited 72.4% sensitivity and 97.2% specificity with 90% accuracy. Among a number of studied variables, diarrhea and colic were significantly associated with microsporidia infection when diagnosed by either technique.
Conclusion
The use of sensitive and discriminative molecular tools will contribute to determining the true prevalence of microsporidiosis and possibly their potential transmission source depending on species identification.




Similar content being viewed by others
References
Simpson AGB, Slamovits CH, Archibald JM (2017) Protist diversity and eukaryote phylogeny. Handbook of the Protists, vol 2. Springer International Publishing, Berlin, pp 1–20. https://doi.org/10.1007/978-3-319-28149-0_45
Carlson J, Helton C, Munn R, Wasson K, Perez R, Gallay B, Finkbeiner WE (2004) Disseminated microsporidiosis in a pancreas/kidney transplant recipient. Arch Pathol Lab Med 128(3):41–42. https://doi.org/10.1043/1543-2165(2004)128<e41:DMIAKT>2.0.CO.2
Viriyavejakul P, Nintasen R, Punsawad C, Chaisri U, Punpoowong B, Riganti M (2009) High prevalence of Microsporidium infection in HIV-infected patients. Southeast Asian J Trop Med Public Health 40(2):223–228
Dikman AE, Schonfeld E, Srisarajivakul NC, Poles MA (2015) Human immunodeficiency virus-associated diarrhea: still an issue in the era of antiretroviral therapy. Digest Dis Sci 60(8):2236–2245. https://doi.org/10.1007/s10620-015-3615-y
Didier ES, Weiss LM (2006) Microsporidiosis: current status. Curr Opin Infect Dis 19(5):458–492. https://doi.org/10.1097/01.qco.0000244055.46382.23
Orenstein JM, Gaetz HP, Yachnis AT, Frankel SS, Mertens RB, Didier ES (1997) Disseminated microsporidiosis in AIDS: are any organs spared? AIDS 11(3):385–386
Garcia LS (2002) Laboratory identification of the microsporidia. J Clin Microbiol 40(6):1892–1901. https://doi.org/10.1128/jcm.40.6.1892-1901.2002
Ali M, Mahmoud LA, Abaza B, Ramadan M (2000) Intestinal spore-forming protozoa among patients suffering from chronic renal failure. J Egypt Soc Parasitol 30(1):93–100
El-Mahallawy H, Zaki MM, El-Arousy M, Shalabi L, Mansour T (2011) Diagnosis of intestinal microsporidiosis in pediatric oncology patients in Egypt using modified acid fast trichrome staining versus PCR. Acta Parasitol 56(4):348–352. https://doi.org/10.2478/s11686-011-0072-4
Kokoskin E, Gyorkos TW, Camus A, Cedilotte L, Purtill T, Ward B (1994) Modified technique for efficient detection of microsporidia. J Clin Microbiol 32(4):1074–1075. https://doi.org/10.1128/JCM.32.4.1074-1075.1994
Raynaud L, Delbac F, Broussolle V, Rabodonirina M, Girault V, Wallon M et al (1998) Identification of Encephalitozoon intestinalis in travelers with chronic diarrhea by specific PCR amplification. J Clin Microbiol 36(1):37–40. https://doi.org/10.1128/JCM.36.1.37-40.1998
Coyle C, Wittner M, Kotler D, Noyer C, Orenstein J, Tanowitz H et al (1996) Prevalence of microsporidiosis due to Enterocytozoon bieneusi and Encephalitozoon (Septata) intestinalis among patients with AIDS-related diarrhea: determination by polymerase chain reaction to the microsporidial small-subunit rRNA gene. Clin Infect Dis 23(5):1002–1006. https://doi.org/10.1093/clinids/23.5.1002
Viera AJ, Garrett JM (2005) Understanding inter observer agreement: the kappa statistic. Fam Med 37(5):360–363
Didier ES (1998) Microsporidiosis. Clin Infect Dis 27(1):1–7. https://doi.org/10.1086/514607
Aini UN, Al-Mekhlafi MH, Anisah N, Fatmah MS, Azlin M, Rozlida AR et al (2008) A preliminary study on the prevalence of intestinal microsporidiosis in patients with and without gastrointestinal symptoms in Malaysia. Trans R Soc Trop Med Hyg 102(12):1274–1278. https://doi.org/10.1016/j.trstmh.2008.05.019
Nadham KM, Maysloon AAS (2012) Microsporidiosis among children with malignant diseases in Basrah, Iraq. Pak J Med Sci 28(4):621–624
Centers of Diseases Control and prevention (2009) Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents. MMWR 58(10):3–59
Muller A, Bialek R, Kamper A, Fatkenheuer G, Salzberger B, Franzen C (2001) Detection of microsporidia in travelers with diarrhea. J Clin Microbiol 39:1630–1632. https://doi.org/10.1128/JCM.39.4.1630-1632.2001
García L, Cano-Estrada A, Cortés-Campos A, Medina-Sansón A, Jiménez-Cardoso E (2013) Identification of Microsporidium spp in patients with acute lymphoblastic leukemia. Boletin Medico del Hospital Infantil de Mexico 70(1):24–28
Kazemi E, Tavalla M, Maraghi S, Jafaryad M, Latifi M (2017) Frequency of microsporidial infection in immunocompromised patients with staining and molecular methods based on internal transcribed spacer region gene in two cities of Southwest Iran during 2013–2014. AJPRHC 9(1):7–16. https://doi.org/10.18311/ajprhc/2017/6014
Fadl HO, El-gabrty SAE, Hanafy NA, Khairy H, Ali N (2015) Detection of microsporidiosis in cases with chronic renal failure. J Life Appl Sci 2(1):43–51
Chabchoub N, Abdelmalek R, Mellouli F, Kanoun F, Thellier M, Bouratbine A et al (2009) Genetic identification of intestinal microsporidia species in immunocompromised patients in Tunisia. Am J Trop Med Hyg 80(1):24–27. https://doi.org/10.4269/ajtmh.2009.80.24
Saigal K, Khurana S, Sharma A, Sehgal R, Malla N (2013) Comparison of staining techniques and multiplex nested PCR for diagnosis of intestinal microsporidiosis. Diagn Microbiol Infect Dis 77(3):248–249. https://doi.org/10.1016/j.diagmicrobio.2013.07.004
Müller AK, Stellermann P, Hartmann M, Schrappe G, Fätkenheuer B, Salzberger V (1999) A powerful DNA extraction method and PCR for detection of microsporidia in clinical stool specimens. Clin Diagn Lab Immunol 6:243–246
Kaushik S, Saha R, Das S, Ramachandran VG, Goel A (2018) Pragmatic combination of available diagnostic tools for optimal detection of intestinal microsporidia. Adv Exp Med Biol 1057:85–94. https://doi.org/10.1007/5584_2017_97
Rinder H, Janitschke K, Aspöck H, Da Silva AJ, Deplazes P, Fedorko D (1998) Blinded, externally controlled multicenter evaluation of light microscopy and PCR for detection of Microsporidia in stool specimens. The diagnostic multicenter study group on microsporidia. J Clin Microbiol 36(6):1814–1818. https://doi.org/10.1128/JCM.36.6.1814-1818.1998
Subrungruang I, Mungthin M, Chavalitshewinkoon-Petmitr P, Rangsin RS, Naaglor T, Leelayoova S (2004) Evaluation of DNA extraction and PCR methods for detection of Enterocytozoon bienuesi in stool specimens. J Clin Microbiol 42(8):3490–3494. https://doi.org/10.1128/JCM.42.8.3490-3494.2004
Mohammed H, Endeshaw T, Kebede A, Defera M (2009) Comparison of chromotrope 2R and Uvitex 2B for the detection of intestinal microsporidial spores in stool specimens of HIV patients attending Nekempte Hospital, West Ethiopia. Ethiop Med J 47(3):233–237
Conteas CN, Berlin OG, Ash LR, Pruthi JS (2000) Therapy for human gastrointestinal microsporidiosis. Am J Trop Med Hyg 63(4–6):121–127. https://doi.org/10.4269/ajtmh.2000.63.121
Kotkova M, Sak B, Kvetonova D, Kvac M (2013) Latent microsporidiosis caused by Encephalitozoon cuniculi in immunocompetent hosts: a murine model demonstrating the ineffectiveness of the immune system and treatment with albendazole. PLoS One 8(4):e60941. https://doi.org/10.1371/journal.pone.0060941
Molina JM, Goguel J, Sarfati C, Michiels JF, Desportes-Livage I, Balkan S et al (2000) Trial of oral fumagillin for the treatment of intestinal microsporidiosis in patients with HIV infection. ANRS 054 Study Group. Agence Nationale de Recherche sur le SIDA. AIDS 14(10):1341–1348. https://doi.org/10.1097/00002030-200007070-00006
Champion L, Durrbach A, Lang P, Delahousse M, Chauvet C, Sarfati C (2010) Fumagillin for treatment of intestinal microsporidiosis in renal transplant recipients. Am J Transplant 10(8):1925–1930. https://doi.org/10.1111/j.1600-6143.2010.03166.x
Larsson JI (2005) Fixation of microsporidian spores for electron microscopy. J Invertebr Pathol 90(1):47–50. https://doi.org/10.1016/j.jip.2005.06.016
De Groote MA, Visvesvara G, Wilson ML, Pieniazek NJ, Slemenda SB, daSilva AJ et al (1995) Polymerase chain reaction and culture confirmation of disseminated Encephalitozoon cuniculi in a patient with AIDS: successful therapy with albendazole. J Infect Dis 171(5):1375–1378. https://doi.org/10.1093/infdis/171.5.1375
Tsai ST, Huang WF, Wang CH (2005) Complete sequence and gene organization of the Nosema spodopterae rRNA gene. J Eukaryot Microbiol 52(1):52–54. https://doi.org/10.1111/j.1550-7408.2005.3291rr.x
Tay W, Mahony EM, Paxton RJ (2005) Complete rRNA gene sequences reveal that the microsporidium Nosema bombi infects diverse Bumblebee (Bombus spp.) hosts and contains multiple polymorphic sites. J Eukaryot Microbiol 52(6):505–513. https://doi.org/10.1111/j.1550-7408.2005.00057.x
Espern A, Morio F, Miegeville M, Illa H, Abdoulaye M, Meyssonnier V et al (2007) Molecular study of microsporidiosis due to Enterocytozoon bieneusi and Encephalitozoon intestinalis among human immunodeficiency virus-infected patients from two geographical areas: Niamey, Niger, and Hanoi, Vietnam. J Clin Microbiol 45(9):2999–3002. https://doi.org/10.1128/JCM.00684-07
Akinbo FO, Okaka CE, Omoregie R (2010) Prevalence of intestinal parasitic infections among HIV patients in Benin City, Nigeria. Libyan J Med 5(1):1–6. https://doi.org/10.3402/ljm.v5i0.5506
Hamamcı B, Çetinkaya Ü, Berk V, Kaynar L, Kuk S (2015) Prevalence of Encephalitozoon intestinalisand Enterocytozoon bieneusi in cancer patients under chemotherapy. Mikrobiyol Bul 49(1):105–113. https://doi.org/10.5578/mb.8787
Polley SD, Boadi S, Watson J, Curry A, Chiodini PL (2011) Detection and species identification of microsporidial infections using SYBR Green real-time PCR. J Med Microbiol 60(4):459–466. https://doi.org/10.1099/jmm.0.026781-0
Childs-Sanford SE, Garner MM, Raymond JT, Didier ES, Kollias GV (2006) Disseminated microsporidiosis due to Encephalitozoon hellem in an Egyptian fruit bat (Rousettus aegyptiacus). J Comp Pathol 134(4):370–373. https://doi.org/10.1016/j.jcpa.2006.01.004
Künzel F, Joachim A (2010) Encephalitozoonosis in rabbits. Parasitol Res 106(2):299–309. https://doi.org/10.1007/s00436-009-1679-3
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shehab, A.Y., Moneer, E.A., Allam, A.F. et al. Intestinal Microsporidia Infection in Leukemic Children: Microscopic and Molecular Detection. Acta Parasit. 66, 346–353 (2021). https://doi.org/10.1007/s11686-020-00283-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11686-020-00283-2