Abstract
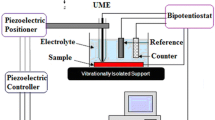
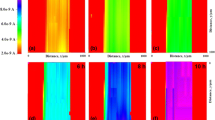
The corrosion protection performances of epoxy-coated Mn steel and carbon steel were evaluated by electrochemical impedance spectroscopy (EIS) and scanning electrochemical microscopy (SECM) analysis. EIS was performed on coated Mn steel with a scratch in a 0.1 M NaCl solution after a wet/dry cyclic corrosion test. The charge transfer resistance (R ct) and film resistance (R f) of the coated Mn steel displayed a higher value than the coated carbon steel. The increase in the charge transfer resistance and film resistance of the coated steel is due to the presence Mn in steel. SECM was conducted to estimate the corrosion protection performance of the epoxy-coated Mn steel immersed in a 0.1 M NaCl solution. It was found that dissolution of Fe2+ was suppressed at the scratch on the coated Mn steel due to the higher resistance for anodic dissolution of the substrate. SEM/EDX analysis showed that Mn was enriched in corrosion products at a scratched area of the coated steel after corrosion testing. FIB-TEM analysis confirmed the presence of the nanoscale oxide layer of Mn in the rust of the steel, which had a beneficial effect on the corrosion resistance of the coated steel by forming protective corrosion products in the wet/dry cyclic test.














Similar content being viewed by others
References
C.F. Dong, H. Sheng, Y.H. An, X.G. Li, K. **ao, Y.F. Cheng, Corrosion of 7A04 aluminum alloy under defected epoxy coating studied by localized electrochemical impedance spectroscopy. Prog. Org. Coat. 67, 269–273 (2010)
S.L. Sinebryukhov, A.S. Gnedenkov, D.V. Mashtalyar, S.V. Gnedenkov, PEO-coating/substrate interface investigation by localised electrochemical impedance spectroscopy. Surf. Coat. Technol. 205, 1697–1705 (2010)
F.J. Maile, T. Schauer, C.D. Eisenbach, Evaluation of the delamination of coatings with scanning reference electrode technique. Prog. Org. Coat. 38, 117–120 (2000)
R. Akid, D.J. Mills, A comparison between conventional macroscopic and novel microscopic scanning electrochemical methods to evaluate galvanic corrosion. Corros. Sci. 43, 1203–1216 (2001)
M. Khobaib, A. Rensi, T. Matakis, M.S. Donley, Real time map** of corrosion activity under coatings. Prog. Org. Coat. 41, 266–272 (2001)
J. He, V.J. Gelling, D.E. Tallman, G.P. Bierwagen, A scanning vibrating electrode study of chromated-epoxy primer on steel and aluminum. J. Electrochem. Soc. 147, 3661–3666 (2000)
K. Fushimi, M. Seo, An SECM observation of dissolution distribution of ferrous or ferric ion from a polycrystalline iron electrode. Electrochim. Acta 47, 121–127 (2001)
J. Bernard, M. Chatenet, F. Dalard, Understanding aluminum behaviour in aqueous alkaline solution using coupled techniques: Part I. Rotating ring-disk study. Electrochim. Acta 52, 86–93 (2006)
K. Fushimi, K.A. Lill, H. Habazaki, Heterogeneous hydrogen evolution on corroding Fe–3 at.% Si surface observed by scanning electrochemical microscopy. Electrochim. Acta 52, 4246–4253 (2007)
T.E. Lister, P.J. Pinhero, The effect of localized electric fields on the detection of dissolved sulfur species from Type 304 stainless steel using scanning electrochemical microscopy. Electrochim. Acta 48, 2371–2378 (2003)
T.E. Lister, P.J. Pinhero, Microelectrode array microscopy: investigation of dynamic behavior of localized corrosion at type 304 stainless steel surfaces. Anal. Chem. 77, 2601–2607 (2005)
J.C. Seegmiller, D.A. Buttry, A SECM study of heterogeneous redox activity at AA2024 surfaces corrosion, passivation, and anodic films. J. Electrochem. Soc. 150, B413–B418 (2003)
J. Izquierdo, J.J. Santana, S. González, R.M. Souto, Uses of scanning electrochemical microscopy for the characterization of thin inhibitor films on reactive metals: the protection of copper surfaces by benzotriazole. Electrochim. Acta 55, 8791–8800 (2010)
F.J. Martin, G.T. Cheek, W.E. O’Grady, P.M. Natishan, Impedance studies of the passive film on aluminium. Corros. Sci. 47, 3187–3201 (2005)
R.M. Souto, Y. Gonzalez-Garcıa, S. Gonzalez, G.T. Burstein, Damage to paint coatings caused by electrolyte immersion as observed in situ by scanning electrochemical microscopy. Corros. Sci. 46, 2621–2628 (2004)
R.M. Souto, Y. González-García, J. Izquierdo, S. González, Examination of organic coatings on metallic substrates by scanning electrochemical microscopy in feedback mode: Revealing the early stages of coating breakdown in corrosive environments. Corros. Sci. 52, 748–753 (2010)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Raj, X.J., Nishimura, T. Evaluation of the Corrosion Protection Performance of Epoxy-Coated High Manganese Steel by SECM and EIS Techniques. J Fail. Anal. and Preven. 16, 417–426 (2016). https://doi.org/10.1007/s11668-016-0102-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11668-016-0102-5