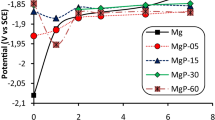
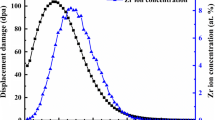
Abstract
Surface attributes of pure Mg such as biocompatibility, mechanical strength and corrosion are clinically important for artificial implants, but its fast degradation post-implantation has drawbacks. Tuning Mg for clinical needs is achieved by techniques like near surface ion implantation. This work is about improving the surface and corrosion rate of bioresorbable magnesium by phosphorous (P) ion implantation with fluences of 1016-1018 ions/cm2 and ion beam accelerating energy of ~ 700 keV by a Pelletron Linear Accelerator. The in vitro degradation rate was examined using electrochemical potential tests in simulated body fluid (SBF). X-ray diffraction and energy-dispersive x-ray spectroscopy confirmed the implantation of P ions by forming an amorphous P-layer on the Mg surface. Surface hardness and electrochemical resistance improved as confirmed by the mechanical and electrochemical tests in SBF, respectively, post-implantation. Medical implants designed by this modified Mg are compliant with the patient’s healing span post-implantation with decreased corrosion rate agreeing with the healing process, avoiding an implant removal surgery post-patient’s recovery.
Graphical Abstract













Similar content being viewed by others
References
H.J.P.I.B. Hermawan, Updates on the Research and Development of Absorbable Metals for Biomedical Applications, Prog. Biomater., 2018, 7(2), p 93–110.
Y. Jia et al., Surface Properties of Magnesium Improved by Sr Ion Implantation, Mater. Res. Exp., 2018, 5(6), p 066546.
S. Somasundaram, M. Ionescu, and B.K.J.M. Mathan, Ion Implantation of Calcium and Zinc in Magnesium for Biodegradable Implant Applications, Multidiscip. Digit. Publ. Inst. (MDPI), 2018, 8(1), p 30.
J. Yang et al., A Review on the Exploitation of Biodegradable Magnesium-Based Composites for Medical Applications, Biomed. Mater., 2018, 13(2), p 022001.
R. Xu et al., Eelectrochemical Properties and Corrosion Resistance of Carbon-Ion-Implanted Magnesium, Corros. Sci., 2014, 82, p 173–179.
N. Kirkland, N. Birbilis, and M.J.A.B. Staiger, Assessing the Corrosion of Biodegradable Magnesium Implants: A Critical Review of Current Methodologies and Their Limitations, Acta Biomater., 2012, 8(3), p 925–936.
Z. **aobo et al., Microstructure, Mechanical and Corrosion Properties of Mg-(4–x) Nd-xGd-Sr-Zn-Zr Biomagnesium Alloys, Sci. Press, 2014, 50(8), p 979–988.
Z.U. Rahman, L. Pompa, and W.J.J.O.M.S.M.I.M. Haider, Electrochemical Characterization and In-Vitro Bio-Assessment of AZ31B and AZ91E Alloys as Biodegradable Implant Materials, J. Mater. Sci. Mater. Med., 2015, 26(8), p 1–11.
Wilson, J.J.F.B.M., Metallic Biomaterials: State of the Art and New Challenges. Fundam. Biomater. Metals, 2018: 1-33
N. Manam et al., Study of Corrosion in Biocompatible Metals for Implants: A Review, J. Alloy. Compd., 2017, 701, p 698–715.
M.A. Shafique et al., Effect of Do** Concentration on Absorbance, Structural, and Magnetic Properties of Cobalt-Doped ZnO Nano-Crystallites, Int. Nano Lett., 2012, 2(1), p 1–7.
M. Nastasi et al., Ion-Solid Interactions: Fundamentals and Applications, Cambridge University Press, Cambridge, 1996.
H. Ryssel and I. Ruge, Ion Implantation, John Wiley & Sons, Chichester, 1986.
J. Muñoz-García et al., Self-organized surface nanopatterning by ion beam sputtering, Toward Functional Nanomaterials. Springer, Berlin, 2009, p 323–398
M. Castro et al., Self-Organized Ordering of Nanostructures Produced by Ion-Beam Sputtering, Phys. Rev. Lett., 2005, 94(1), p 016102.
S.U. Awan et al., Correlation Between Structural, Electrical, Dielectric and Magnetic Properties of Semiconducting Co Doped and (Co, Li) Co-Doped ZnO Nanoparticles for Spintronics Applications, Physica E, 2018, 103, p 110–121.
S.U. Awan et al., Room Temperature p-Type Conductivity and Coexistence of Ferroelectric Order in Ferromagnetic Li Doped ZnO Nanoparticles, J. Appl. Phys., 2014, 116(16), p 164109.
N. Ali et al., Assessment of Fatigue and Corrosion Fatigue Behaviours of the Nitrogen Ion Implanted CpTi, Int. J. Fatigue, 2014, 61, p 184–190.
K. Feng et al., Improved Corrosion Resistance of Stainless Steel 316L by Ti Ion Implantation, Mater. Lett., 2012, 68, p 450–452.
P. Tian and X.J.R.B. Liu, Surface Modification of Biodegradable Magnesium and its Alloys for Biomedical Applications, Regen. Biomater., 2015, 2(2), p 135–151.
H. Wang et al., Improvement of In Vitro Corrosion and Cytocompatibility of Biodegradable Fe Surface Modified by Zn Ion Implantation, Appl. Surf. Sci., 2017, 403, p 168–176.
M. Esmaily et al., Fundamentals and Advances in Magnesium Alloy Corrosion, Prog. Mater Sci., 2017, 89, p 92–193.
A. Adhilakshmi, K. Ravichandran, and S.N.J.N.J.O.C. Tsn, Protecting electrochemical degradation of pure iron using zinc phosphate coating for biodegradable implant applications, New J. Chem., 2018, 42(22), p 18458–18468.
X. Wei et al., Improvement on Corrosion Resistance and Biocompability of ZK60 Magnesium Alloy by Carboxyl Ion Implantation, Corros. Sci., 2020, 173, p 108729.
S. Bagherifard et al., Effects of Nanofeatures Induced by Severe Shot Peening (SSP) on Mechanical, Corrosion and Cytocompatibility Properties of Magnesium Alloy AZ31, Acta Biomater., 2018, 66, p 93–108.
X.W. Tao et al., Nanomechanical and Corrosion Properties of zk60 Magnesium Alloy Improved by gd Ion Implantation, Surf. Rev. Lett., 2014, 21(06), p 1450085.
Z. Wang et al., Corrosion Behaviour of Nd Ion Implanted Mg–Gd–Zn–Zr Alloy in Simulated Body Fluid, Adv. Perform. Mater., 2015, 30(6), p 321–326.
G. Wu et al., Retardation of Surface Corrosion of Biodegradable Magnesium-Based Materials by Aluminum Ion Implantation, Appl. Surf. Sci., 2012, 258(19), p 7651–7657.
M. Lei et al., Wear and Corrosion Resistance of Al Ion Implanted AZ31 Magnesium Alloy, Surf. Coat. Technol., 2007, 201(9–11), p 5182–5185.
C. Liu et al., Corrosion Resistance of Titanium Ion Implanted AZ91 Magnesium Alloy, J. Vac. Sci. Technol. A, 2007, 25(2), p 334–339.
C. Liu et al., Corrosion Behavior of AZ91 Magnesium Alloy Treated by Plasma Immersion Ion Implantation and Deposition in Artificial Physiological Fluids, Thin Solid Films, 2007, 516(2–4), p 422–427.
G. Wan et al., Corrosion Properties of Oxygen Plasma Immersion Ion Implantation Treated Magnesium, Surf. Coat. Technol., 2007, 201(19–20), p 8267–8272.
A. Bakkar and V.J.C.S. Neubert, Improving Corrosion Resistance of Magnesium-Based Alloys by Surface Modification with Hydrogen by Electrochemical Ion Reduction (EIR) and by Plasma Immersion Ion Implantation (PIII), Corros. Sci., 2005, 47(5), p 1211–1225.
X.B. Tian et al., Corrosion Resistance Improvement of Magnesium Alloy Using Nitrogen Plasma Ion Implantation, Surf. Coat. Technol., 2005, 198(1–3), p 454–458.
R. Asri et al., Corrosion and Surface Modification on Biocompatible Metals: A Review, Mater. Sci. Eng., C, 2017, 77, p 1261–1274.
D. Krupa et al., Effect of Phosphorus-Ion Implantation on the Corrosion Resistance and Biocompatibility of Titanium, Biomaterials, 2002, 23(16), p 3329–3340.
D. Krupa et al., Effect of Dual Ion Implantation of Calcium and Phosphorus on the Properties of Titanium, Biomaterials, 2005, 26(16), p 2847–2856.
S. Ferdjani et al., Phosphorus Implantation in Titanium: Application to Calibration Analysis, J. Alloy. Compd., 1991, 177(2), p 265–272.
S. Baunack et al., Depth Distribution and Bonding States of Phosphorus Implanted in Titanium Investigated by AES, XPS and SIMS, Surf. Interface Anal., 1998, 26(6), p 471–479.
E. Wieser et al., Modification of Titanium by Ion Implantation of Calcium and/or Phosphorus, Surf. Coat. Technol., 1999, 111(1), p 103–109.
H.A. Acciari et al., Surface Modifications by Both Anodic Oxidation and Ion Beam Implantation on Electropolished Titanium Substrates, Appl. Surf. Sci., 2019, 487, p 1111–1120.
A. Panepinto, D. Cossement, and R. Snyders, Experimental and Theoretical Study of the Synthesis of N-Doped TiO2 by N Ion Implantation of TiO2 Thin Films, Appl. Surf. Sci., 2021, 541, p 148493.
M. Hashmi et al., Effect of Different CaO/MgO Ratios on the Structural and Mechanical Properties of Bioactive Glass-Ceramics, J. Ceram.-Silikáty, 2012, 56(4), p 347–351.
I. Ismail et al., Determination of the Crystallite Size and Crystal Structure of Magnesum Powder by X-Ray Diffraction, J. Nat., 2020, 20(3), p 61–65.
Q. Liu et al., Crystalline Red Phosphorus Nanoribbons: Large-Scale Synthesis and Electrochemical Nitrogen Fixation, Angew. Chem., 2020, 59(34), p 14383–14387.
M. Hashmi and S.A.J.P.I.N.S.M.I. Shah, Dissolution Behavior of Bioactive Glass Ceramics with Different CaO/MgO Ratios in SBF-K9 and r-SBF, Prog. Nat. Sci. Mater. Int., 2014, 24(4), p 354–363.
F. Aumayr et al., Single Ion Induced Surface Nanostructures: A Comparison Between Slow Highly Charged and Swift Heavy Ions, J. Phys. Condens. Matter, 2011, 23(39), p 393001.
S. Habenicht, W. Bolse, and K.-P.J.R.O.S.I. Lieb, A Low-Energy Ion Implanter for Surface and Materials Science, Rev. Sci. Instrum., 1998, 69(5), p 2120–2126.
Lin, C.-D., Review of fundamental processes and applications of atoms and ions. books.google.com, 1993
C. Lemell et al., Image Acceleration of Highly Charged Ions by Metal Surfaces, Phys. Rev. A, 1996, 53(2), p 880.
C. Lemell et al., On the Nano-Hillock Formation Induced by Slow Highly Charged Ions on Insulator Surfaces, Solid-State Electron., 2007, 51(10), p 1398–1404.
L. Ali et al., High-Quality Optical Ring Resonator-Based Biosensor for Cancer Detection, IEEE Sens. J., 2019, 20(4), p 1867–1875.
A. El-Said et al., Potential Energy Threshold for Nano-Hillock Formation by Impact of Slow Highly Charged Ions on a CaF2 (1 1 1) Surface, Nucl. Instrum. Methods Phys. Res. Sect. B, 2007, 258(1), p 167–171.
A.J.T. Kand et al., Electrochemical Evaluation of the Hydroxyapatite Coating Synthesized on the AZ91 by Electrophoretic Deposition Route, Synth. Sinter., 2021, 1(2), p 85–91.
A.T. Tabrizi et al., Tribological Characterization of Hybrid Chromium Nitride Thin Layer Synthesized on Titanium, Surf. Coat. Technol., 2021, 419, p 127317.
A.T. Tabrizi et al., Correction of Archard Equation for Wear Behavior of Modified Pure Titanium, Tribol. Int., 2021, 155, p 106772.
M. Rahman et al., Microroughness Induced Biomimetic Coating for Biodegradation Control of Magnesium, Mater. Sci. Eng. C, 2021, 121, p 111811.
Acknowledgments
The authors acknowledge the technical assistance of the School of Biomedical Engineering and Health Sciences, Universiti Teknologi Malaysia, National Center for Physics Islamabad, and GIK Institute of technology Topi, Pakistan.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Asdi, M.H., Khan, M.U.A., Hussain, J. et al. Effect of Phosphorous Ion Implantation on the Surface, Crystal Structure, Mechanical, and Electrochemical Properties of Bioresorbable Magnesium for Biomedical Applications. J. of Materi Eng and Perform 31, 7695–7704 (2022). https://doi.org/10.1007/s11665-022-06763-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-022-06763-y