Abstract
The influence of conventional and accumulative HPT (Acc. HPT) process on structural properties of Zr62Cu22Al10Fe5Dy1 metallic glass (MG) phase is examined by X-ray diffraction (XRD) technique. The XRD results showed that HPT process leads to free volume generation in MG. Free volume increases with the number of anvil turns in the conventional HPT process, while the highest free volume increment was observed in Acc. HPT process. To understand the role of latent crystalline phases on free volume, thermal stability and crystallization kinetics of Zr62Cu22Al10Fe5Dy1 MG are investigated by non-isothermal heating using differential scanning calorimetry (DSC). Thermal investigation indicates Dy addition results in increased activation energy along with lower nucleation rate during crystallization. As the initial amorphous phase significantly influences crystallization behavior, d-spacing analysis of amorphous hump is carried out to predict latent crystalline phases. Strong correlation was observed between latent crystalline phases and intermetallic compounds in devitrified Zr62Cu22Al10Fe5Dy1 alloy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Zr-based metallic glasses (MGs) with excellent yield strength, high elastic limit, good corrosion resistance, and biocompatibility are potential materials for biomedical applications.[1,2,3] However, to improve glass forming ability (GFA), toxic elements such as Ni and Be are added in a large number of Zr-based MGs.[4,5,6] Consequently, develo** Zr-based MGs free of such toxic elements is advantageous for biomedical applications. In recent studies, Ni-Free Zr–Cu–Al–Fe MGs have shown excellent GFA.[7,8] Zhang et al.[8] reported Zr62.5Cu22.5Al10Fe5 with good GFA in Zr–Cu–Al–Fe system. Further, influence of Dy addition on GFA in Zr–Cu–Al–Fe system is thoroughly investigated.[9,10] Churyumov et al.[10] demonstrated the improvement in GFA of Zr62.5Cu22.5Al10Fe5 MG with minor addition of Dy, which also resulted in enhanced compressive strength.
Exceptional combination of properties in Zr-based MGs is compromised with major drawback arising due to limited room temperature plastic deformation.[11] The highly localized mode of plastic deformation in MGs takes place in narrow regions (~ 10 nm) known as shear bands.[12] Larger deformation leads to rapid and unrestrained shear band propagation that ultimately results into catastrophic failure. Several studies have shown tailoring of structure in glassy alloys by introducing chemical and physical heterogeneity in the form of clusters, nanocrystals, and shear bands that results in the improvement of ductility in MGs.[13,14,15,16] Introduction of heterogeneities influences the nucleation and propagation of shear bands. High-pressure torsion (HPT) technique is a severe plastic deformation (SPD) method for the processing of metallic materials.[17] Earlier investigations have reported the improvement in ductility of MGs after HPT process.[18] Recently, HPT has been widely considered to transform the structure and properties of glassy alloys due to SPD.[5,19,20,21] HPT process structurally modifies the material without introducing failure because of high hydrostatic pressure along with geometric constrains.[5] Key mechanism for the enhancement in ductility in MGs is due to structure modification leading to an increase in average interatomic spacing which assists nucleation and propagation of shear bands.[22] Recent studies have shown[20] that the actual achievable strain with HPT processing of MG is significantly lower than the one determined theoretically using empirical relation (γ = 2πnR/h).[17] Such inconsistency can be explained by the phenomenon of “slippage” that takes place during the HPT process. To achieve higher strain in hard materials, like MGs, the authors[20] reported a new technique called accumulative HPT (Acc. HPT). The present work reports the influence of conventional and Acc. HPT on Zr62Cu22Al10Fe5Dy1 MG sample at room temperature. The effect of HPT on the free volume generation was analyzed using XRD technique and compared with non-deformed melt spun ribbon of the same composition.
Thermodynamically metastable nature of MGs aids the crystallization process during mechanical deformation and annealing process.[23,24] Previous investigations have reported nanocrystallization during the HPT process of MGs.[25,26,27,28] Nucleation of crystalline phases may be detrimental to mechanical properties of MG. Therefore, understanding of thermal stability and crystallization kinetics of MGs is crucial to prevent and control the nucleation of nanocrystals during HPT process. DSC has been employed to investigate the crystallization kinetics and to extract relevant information like nucleation, growth mechanism, and activation energy.[29,30,31] Numerous investigations have reported crystallization kinetics analysis based on non-isothermal condition.[29,32,33,34,35,36] Peng et al.[37] reported activation energy of Zr60Cu25Al10Fe5 MG determined by non-isothermal heating and studied the crystallization behavior by using isothermal method. Similarly, Luo et al.[35,36] investigated the crystallization kinetics of Zr63Cu23Al11.5Fe2.5 and Zr60Cu20Al10Fe10 MGs under non-isothermal condition. These studies indicate that the crystallization behavior for Zr60Cu20Al10Fe10 MG is nucleation controlled for 80 pct of the process and growth controlled for the remaining 20 pct according to Local Avrami’s exponent (LAE) analysis obtained by Johnson–Mehl–Avrami–Kolmogorov (JMAK) equation, while Zr63Cu23Al11.5Fe2.5 MG showed complete nucleation-dominated crystallization process. To understand the influence of Dy addition on the thermal stability and GFA in Zr–Cu–Al–Fe MG, crystallization behavior of Zr62Cu22Al10Fe5Dy1 MG is investigated by using non-isothermal condition. Further, structural analysis of thermally treated sample was carried out using XRD to correlate the formation of intermetallic phases obtained from d-spacing analysis.
2 Experimental Details
Master alloy of Zr62Cu22Al10Fe5Dy1 composition is synthesized by arc melting of pure Zr, Cu, Al, Fe, and Dy (> 99.9 mass pct) in argon atmosphere. Melt spinning process is used to produce ribbon of 0.04 mm thickness and ~ 2 mm width from the master alloy using single copper roller. Further ribbon is equally cut into segments and three precompact samples are produced by using press mold of 10 mm diameter under 100 MPa pressure. Two precompact samples are subjected to conventional HPT processing under 6 GPa compressive pressure and anvil rotation of 1 rpm. First precompacted sample is subjected 2 anvil rotation (MS-02) and second sample to 5 anvil rotations (MS-05). Third precompacted sample (MS-11) is processed by Acc. HPT. Throughout the Acc. HPT process, compressive pressure of 6 GPa and rotational speed of 1 rpm is maintained. Acc. HPT process can be divided into three stages. Precompacted sample is subjected to 1 anvil rotation during first stage. During second stage, sample is cut into four segments. These segments are stacked between anvil and subjected to 5 anvil rotations. For the third stage same process, as second stage, is repeated to produce solid disk of 10 mm diameter and 0.4 mm thickness.
X-ray diffraction (XRD) analysis of melt spun ribbon (MS-0), MS-02, MS-05, MS-11, and annealed samples are carried out using Bruker D8 advance ECO with CuKα source (λ = 1.54 Å) with step size of 0.02 deg and time per step of 0.6 seconds. DSC analysis of MS-0 was performed using Labsys Evo TG-DSC 1600 °C under argon atmosphere at different heating rates (10, 20, 30, and 40 K/min). High-resolution transmission microscopy (HRTEM) investigation of annealed Zr62Cu22Al10Fe5Dy1 samples is carried out using Tecnai G2, F30 microscope operated at 300 kV. Indexing of selected area electron diffraction (SAED) pattern is carried out using software CrystBox.[38]
3 Results and Discussion
3.1 XRD Analysis
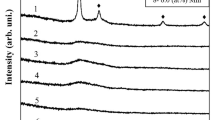
XRD analysis of melt spun ribbon and HPT-processed MG samples are carried out to investigate the structural changes occurred during SPD as shown in Figure 1(a). XRD curve for MS-0 showed broad diffused humps and absence of any sharp crystalline peaks confirming the amorphous nature of the sample before HPT process. The absence of sharp Bragg’s peak in the XRD pattern for MS-02, MS-05, and MS-11 also indicates the retention of amorphous structure after HPT process. Therefore, in the present study, no tangible contribution from stress-induced crystallization is observed in HPT-processed MGs which otherwise are reported in the previous investigations.[25,26,27,28] XRD results (Figure 1(a)) also showed secondary amorphous hump of lower intensity along with primary hump. Earlier reports indicate that the additional secondary hump is related to the presence of short-range ordering (SRO) in the amorphous structure.[39,40]
For further analysis, peak fit for XRD curve is carried out using pseudo-Voigt function[41] to obtain maxima “2θ” position of diffused primary hump as shown in Figure 1(a). Structural parameters related to XRD analysis are listed in Table I. Determination of d-spacing and radius of the first coordination sphere (R1) is carried out using Eqs. [1] and [2].[42]
where θ is the scattering angle and λ is the wavelength of X-ray radiation. The relative change in free volume (∆V) after HPT process can be calculated from the R1 according to Eq. [3] proposed by Gunderov et al.[42] using bulk XRD technique:
where R0 and RHPT are the radii of first coordination sphere for MS and HPT samples. From Table I, it is observed that the first amorphous hump shifts towards lower diffracting angle after HPT process. This shift in position is due to increase in the radius of first coordination sphere as a result of creation in free volume (Figure 1(b)) during SPD.[43] During conventional HPT process, 0.09 pct increase in the free volume (∆V) after 2 revolutions and further 0.71 pct increase after 5 revolutions is an indication of strong influence by plastic deformation. Change in free volume is highest (∆V = 0.87 pct) for the Acc. HPT-processed sample (MS-11) could be due to severity in plastic deformation as compared to conventional HPT process.[20]
Lack of long-range atomic ordering in MG restricts plastic strain distribution. Park[44] proposed the distribution of free volume among the dense region made up of atomic clusters. Empty spaces (free volume) have lower coordination number as compared to dense clusters. Interestingly, Ding et al.[45] identified free volume region as topologically unstable that aids the rearrangement of atoms during plastic deformation to form shear transformed zone (STZ). It is understood that the increase in applied strain results in the formation of continuous STZ in the direction of resolved shear strain. The formation of large STZ leads to nucleation of shear bands, which is the primary mode for plastic deformation in MGs. Alternatively, it is demonstrated that the increase in free volume improves the ductility of the MG after HPT process.[22]
To further understand the effect on deformation behavior due to the presence of small-scale chemical structural units, the d-spacing analysis for the latent crystalline phases that lie under the primary hump is carried out as shown in Figures 2(a) and (b) and listed in Table II. The d-spacing value for HPT-treated samples is different due to varying deformation conditions. Variations in d-spacing values indicate the change in nature of the SRO[46] resulting from HPT process. Earlier studies have reported that d-spacing analysis is useful to determine interplanar spacing of phases present in devitrified MG.[47] In the same study, similarity in R1 value is reported for results obtained from conventional and high-energy XRD. Nucleation of certain prominent crystalline phases obtained by annealing of MG could be connected to the latent crystalline phases that contribute to the formation of specific amorphous hump observed in XRD pattern. Interestingly, prominent binary phases that are formed among Zr, Cu, Al, Fe, and Dy have their d-spacing values (Table III) very similar to values reported for primary hump (Table II). Such observations give an indication of strong resemblance between small chemical structural unit under amorphous hump and fully grown crystalline phases in devitrified state. To address this, chemical enthalpy (ΔH) values[9] are calculated for the observed intermetallic phases based on d-spacing analysis and is represented in a form of bar diagram indicating bonding strength between dominant binary pairs as shown in Figure 2(c).
Previous investigators have highlighted the importance of quenched-in nuclei that significantly affect the crystallization kinetics,[46,48,49,50,51] which is also supported by the presence as SRO in Zr60Cu20Pd10Al10 MG.[46] Large negative ΔH promotes the formation of stable quenched-in nuclei clusters during the annealing of MG and their tentative pathway is depicted in Figure 3. Topological and chemical characteristics of the quenched-in nuclei are dependent on the local atomic environment which is basically governed by interaction among the participating atoms.[46] Localized rise in temperature during HPT process cannot be neglected and may also result in the formation of quenched-in nuclei in MG matrix.[52] Therefore, understanding the nature of quenched-in nuclei which largely interferes with shear bands due to similarity in their dimension could assist to decipher the deformation behavior of HPT-processed samples. To compare our observation, we have taken the crystalline phases reported during the annealing of Zr-rich MG such as Zr55Cu45, Zr60Cu30Al10, Zr62.5Cu22.5Al10Fe5 that are tabulated in Table IV.[53,54] It was noticed that Zr2Cu phase formed in all the above three MGs, while Cu10Zr7 formed only in Zr55Cu45 and a possible ternary phase of Al–Cu–Zr formed in other two, i.e., Zr60Cu30Al10, Zr62.5Cu22.5Al10Fe5 MG. This indicates that Zr2Cu is the most prominent crystalline phase formed during the devitrification of Zr–Cu (Al, Fe) MGs.
3.2 Non-isothermal Crystallization Analysis
As discussed earlier, understanding of the thermal stability of phases in MG is vital to understand the structure–property correlation after HPT process in the same sample. To understand crystallization kinetics by thermal means, non-isothermal study is carried out using DSC technique for Zr62Cu22Al10Fe5Dy1 MG at different heating rates as shown in Figure 4(a). The characteristic glass transition (Tg), onset for first and second crystallization (Tx1 and Tx2) temperatures are determined and tabulated in Table V. It is observed that Tg, Tx1, and Tx2 shift towards the higher temperatures with the increase in heating rate. Characteristic temperature dependence on heating rate is a well-known phenomenon that describes the mechanism of crystallization process due to thermal activation aided by structural relaxation to form stable crystalline phases.[55] The addition of Dy remarkably changes the nature of thermogram with two exothermic peak in Zr62Cu22Al10Fe5Dy1 MG (Figure 4(a)) otherwise only one peak is reported for Zr62.5Cu22.5Al10Fe5 MG.[53] Thermodynamic basis for such behavior can be attributed to chemical interaction resulting in high negative ΔH values between the binary pairs. In the present composition, dominant interaction of Dy with Cu and Al results in chemical SRO in the MG (ΔHDy–Al = − 39 kJ/mol and ΔHDy–Cu = − 22 kJ/mol) that primarily influences the crystallization behavior .[56,57]
Figures 4(b) through (e) show deconvolution of crystallization peaks at various heating rates. Figure 4(f) shows the XRD pattern for Zr62Cu22Al10Fe5Dy1 MG annealed at 755 K and 845 K for 1200 seconds under argon atmosphere. ΔH and melting temperature for crystalline phases identified during XRD analysis of isothermally treated samples are listed in Table VI.[58] During the isothermal heating at 755 K, crystallization of three different phases were observed viz., Zr3Al2, Cu10Zr7, and Zr2Al. Interestingly, the presence of Zr2Al and Zr3Al2 phases in latent form was conformed in d-analysis of primary amorphous hump (Table II). This establishes strong connection between the latent crystalline phases in amorphous hump and devitrified phases. Further, crystallization of Cu10Zr7 phase was observed instead of Zr2Cu phase contrary to the reported results (Table IV). Probable reason for such observation is the separation of Zr and Cu in amorphous phase with Al distributed uniformly as reported in earlier studies for Zr60Cu30Al10 MG.[59] Separation results in the formation of Cu-rich and Zr-rich cluster in the amorphous phase.[60] The presence of Cu-rich cluster aids in the crystalline phases formation of Cu10Zr7. Further in Zr-rich clusters, nucleation of higher ΔH phases like Zr3Al2 and Zr2Al are preferred over Zr2Cu. XRD analysis of sample annealed at 855 K showed that alloy is composed of multiphases. These phases were identified as Zr2Al3, Zr2Cu, ZrAl, Cu10Zr7, and Zr2Al. Comparing the results of sample annealed at 755 K and 845 K, it can be concluded that Zr3Al2 is metastable phase formed at 755 K and is transformed into Zr2Al3 + ZrAl at 845 K. Earlier study has also reported in the crystallization of metastable Zr3Al2 phase in Zr69.5Cu12Ni11Al7.5 MG.[61] Further, Zr2Cu phase may have precipitated directly from retained amorphous phase but crystallization pathway is unclear. Figure 5 shows the HRTEM images with SAED pattern (inset) of Zr62Cu22Al10Fe5Dy1 samples annealed at 755 K (a and b) and at 847 K (c and d). Lattice fringe spacing and analysis of SAED (inset) showed crystallite in Figure 5(a) corresponds to that of Zr3Al2 phase. Further, lattice fringe spacing and SAED pattern for crystallites shown in Figure 5(b) match the Cu10Zr7 and Zr2Al phases. Similarly, lattice fringe spacing and SAED pattern for sample annealed at 847 K is also analyzed. Crystallites in Figures 5(c) and (d) are identified as Zr2Cu and Zr2Al3, respectively. The presence of latent crystalline phases in the form of SRO may contribute to the intensity, position, and broadening of amorphous hump in XRD profile. Contribution in “2θ” broadening in XRD profile is also reported for nanocrsytals in devitrified MG.[59] Transformation of SRO-dominated latent crystalline phases to long-range order crystalline phase will take place with the influence of thermal energy as observed in DSC. Similar phenomenon can also be connected for mechanical activation energy through HPT. Coexistence of all the five crystalline phases in different proportion is an indication of competition that is governed by three factors, i.e., elemental composition, thermodynamics among constituents, and atom topological characteristics. Further, the presence of multiple phases in devitrified MG indicates nucleation dominance during crystallization process.
To understand crystallization kinetics, the activation energy (EC) at onset temperature for first and second crystallization peaks was evaluated by Kissinger’s method[62] using the following equation:
where β is heating rate, T is characteristic temperature, R is universal gas constant, and C is a fitting constant obtained from Kissinger’s plot. The Kissinger’s plot, i.e., ln(β/T2) vs. 1000/T for first and second crystallization peaks are plotted as shown in Figure 6(a). From Kissinger’s plot, activation energy calculated for first (EC1) and second crystallization peaks (EC2) is 379.45 and 676.09 kJ/mol, respectively.
(a) Kissinger plot’s for Tx1 and Tx2, non-isothermal crystallization analysis of Zr62Cu22Al10Fe5Dy1 metallic glass: (b) plot of crystallization volume fraction (x) vs. temperature, (c) Kissinger plot for different crystallized volume fractions (x), and (d) plot of crystallization activation energy as a function of crystallized volume fraction (x) for first crystallization peak
Assuming crystallization process corresponds to heat release, crystallization volume fraction (x) is proportional to area fraction under the exothermic peak and can be determined using the following equation[63]:
where To is onset and T∞ is offset crystallization temperature, (dHC/dT) is heat capacity at constant temperature, H(T) is area under exothermic peak from onset to temperature T, and H∞ total area under the exothermic peak. Variation of ‘x’ with temperature for different heating rates are shown in Figure 6(b).
As activation energy varies during the non-isothermal crystallization process, analysis of the local activation energy is essential to understand kinetics more precisely. The local activation energy for the first step of crystallization is determined by Kissinger’s method (Eq. [4]). Using temperatures at the corresponding ‘x’ value obtained from Figure 6(b), ln(β/T2) vs. 1000/T for all the x values (0 to 1) are plotted as shown in Figure 6(c). Local activation energies are obtained from slopes in Figure 6(c). Variation of local activation energy with respect to ‘x’ is plotted in Figure 6(d).
From Figure 6(d), during the initial crystallization process rapid decrease in activation energy is observed from 379.94 to 354.04 kJ/mol up to x = 0.1. On further crystallization, local activation energy shows linear descending trend with increase in ‘x.’ Crystallization process of Zr62Cu22Al10Fe5Dy1 MG can be divided into two stages, first stage from x = 0 lasting upto 0.1 and second stage starting from x = 0.1 till the end of first crystallization process. During the initial stage of crystallization, higher activation energy for first peak (379.94 kJ/mol) corresponds to initiation of nucleation. Therefore, initial crystallization process (x < 0.1) is dominated by nucleation. Linear decreasing trend shown during second stage of crystallization observed in the analysis of first peak is due to the inherent nature of activation energy that is summation of two component, related to nucleation and growth. Progression of crystallization results in gradual lowering of activation energy required for nucleation.
Crystallization mechanism is often analyzed by JMAK equation[29,64,65]:
where n is Avrami’s exponent, x(t) is crystallization volume fraction at time t, and K is the reaction rate constant. Arrhenius equation is often used to determine K:
where Ko is constant. During crystallization, rate of nucleation and growth does not remain constant, LAE provides better understanding of variation in nucleation and growth during crystallization process. LAE can be determined by the following equation[34,35]:
The values of n(x) for first crystallization peak are obtained from Eq. [8] by using slope of tangent of ln[-ln(1 − x)] vs. 1000/T plot (Figure 7(a)) and LAE. Variation of average n(x) values with crystallization volume fraction (x) can be observed in Figure 7(b). For this plot average is calculated by considering the n(x) values for various heating rates at corresponding fraction crystallization.
To further understand crystallization behavior, diffusion-controlled growth theory is applied[65] in present study. According to this theory, n(x) value less than 1.5 signifies growth of crystalline phase without any nucleation. For n(x) = 1.5 implies growth of the crystalline phase with close to zero nucleation rate. Further when value n(x) lies between 1.5 and 2.5, growth of the crystalline phase occurs with decreasing nucleation rate. Lastly, n(x) > 2.5 corresponds to growth with increased nucleation rate. From Figure 7(b), it can be observed that n(x) rapidly decreases up to x = 0.3 to the value of below 2.5 [n(x) = 2.44]. From x = 0.3 [n(x) = 2.44] to 0.6 [n(x) = 1.78], a slight dip in n(x) value is observed. During further crystallization [i.e., n(x) > 0.6], minor increase in n(x) value is observed. From the view of diffusion-controlled growth mechanism, up to x = 0.3 crystallization takes place with increasing nucleation rate. During further crystallization, growth of the nuclei continues with decreasing nucleation rate. The value of n(x) remains above 1.5 during the crystallization process indicating that nucleation is a dominant process. For a multicomponent system in supercooled liquid state, large number of SRO clusters exists, that can behave as an overcritical nucleus.[37] During crystallization, simultaneous atomic rearrangement is required to create new nuclei and growth of existing overcritical nuclei. Dense random atomic packing in multicomponent MG restrains diffusion of atoms to retard the rate of nucleation and growth. Hence, decrease in nucleation rate is observed during the first half of crystallization process. It is expected that the growth of initial nuclei during crystallization changes the composition in surrounding matrix. Disturbance by composition fluctuations promotes nucleation in the vicinity close to growing nuclei during latter half of first crystallization process, which results in increased nucleation rate.
Similar to the first crystallization peak, variation of crystallization fraction (x) with respect of temperature for second crystallization peak is determined using Eq. [5] and plotted in Figure 8(a). The local activation energy is calculated by Kissinger’s method[62] using Eq. [4] as shown in Figure 8(b). Variation of local activation energy with respective to x is plotted in Figure 8(c). Local activation energy plot can be divided into three stages. Drastic decrease of activation energy is observed in the first stage from 676.09 and 549.36 kJ/mol till x = 0.1. During second stage, linear decrease activation energy is observed from 581.54 kJ/mol (x = 0.2) to 485.60 kJ/mol (x = 0.9). Steep decrease in activation energy from 485.59 kJ/mol (x = 0.9) to 449. 39 kJ/mol (x = 1.0) in the final stage. Similar trend activation energy is observed in the first crystallization except for the steep decrease at the end of second crystallization process.
(a) Non-isothermal analysis for second crystallization peak of Zr62Cu22Al10Fe5Dy1 metallic glass: (a) plot of crystallization volume fraction (x) vs. temperature, (b) Kissinger plot for different crystallized volume fractions (x), and (c) plot of crystallization activation energy as a function of crystallized volume fraction (x) based on Kissinger method
Using of the slope of tangent for ln[− ln(1 − x)] vs. 1000/T plot (Figure 9(a)) and local activation energy, n(x) values for second crystallization peak are determined from Eq. [8]. Average n(x) values vs. ‘x’ are plotted as shown in Figure 9(b). During the initial stages of second crystallization process (till x = 0.2), n(x) value is observed to lie above 1.5. According to diffusion-controlled growth theory,[65] crystallization till x = 0.2 is dominated by growth nuclei with decrease in nucleation rate. While n(x) value for the rest of process is below 1.5, which suggests crystallization process involves the growth of existing nuclei with no further nucleation. This can be correlated with the variation in the local activation energy in second crystallization process. Higher activation energy observed during initial stages in the second crystallization process is due to nucleation and growth process. Further, the linear decrease in the activation energy can be related to decrease in the growth rate as observed from n(x) values.
The activation energy for the reported Zr–Cu–Al–Fe and Zr62Cu22Al10Fe5Dy1 MGs are listed in Table VII.[35,36,37] For the comparison purpose, only the composition close to Zr62Cu22Al10Fe5Dy1 is chosen from Zr–Cu–Al–Fe system. From Table VII, it can be observed that activation energy for Zr62Cu22Al10Fe5Dy1 MG is much larger as compared to reported Zr–Cu–Al–Fe MGs. Higher activation energy implies that glassy phase in Zr62Cu22Al10Fe5Dy1 MG is more stable as compared to reported Zr–Cu–Al–Fe MGs. Therefore, from the above discussion it is inferred that Dy addition enhances the thermal stability of glassy phase and thereby GFA of Zr–Cu–Al–Fe MGs. This is in accordance with the previous studies that has reported improvement in GFA due to Dy addition in Zr–Cu–Al–Fe MGs.[9,10] Higher thermal stability of the Zr62Cu22Al10Fe5Dy1 MG prevents crystallization during HPT process. Hence, no visible crystalline phases are observed in HPT-processed samples during XRD analysis (Figure 1(a)). Crystallization analysis of Zr62Cu22Al10Fe5Dy1 MG showed growth with decreased nucleation rate-dominated crystallization process, which is in contrast from Zr60Cu20Al10Fe10, Zr60Cu25Al10Fe5 and Zr63Cu23Al11.5Fe2.5 MGs that showed growth with increased nucleation rate.[35,36,37] The above observations may be due to the effect of Dy addition in Zr–Cu–Al–Fe MGs. As discussed earlier, larger negative chemical enthalpy values of Dy with Al and Cu enhance the SRO in the MG.[56,57] Further, covalent diameter of Dy (3.18 Å) is larger in comparison to Cu (2.34 Å), Fe (2.34 Å), Zr (2.9 Å), and Al (2.5 Å), which increases mismatch entropy resulting in dense random atomic structure.[9,56,57] Both stronger SRO and dense structure restrict the diffusion of atoms required for nucleation and thereby resulting in the decrease of nucleation rate with larger activation energy.
4 Conclusions
-
Change in free volume is observed with th increase in the number of rotations in HPT process.
-
Highest increment in free volume is observed for Acc. HPT-processed sample.
-
Improvement in GFA of Zr–Cu–Fe–Al MGs with Dy addition is reflected from the current investigation with higher activation energy for Zr62Cu22Al10Fe5Dy1 MG as compared to the previous reported Zr60Cu20Al10Fe10, Zr60Cu25Al10Fe5 and Zr63Cu23Al11.5Fe2.5 MGs.
-
From crystallization analysis, it is observed that Dy addition hampers the nucleation rate during crystallization process.
-
Crystallization pathway is attempted in Zr62Cu22Al10Fe5Dy1 MG and investigated by isothermal heating. Zr2Al, Zr3Al2, and Cu10Zr7 intermetallic phases precipitated from amorphous phase during isothermal heating at 755 K.
-
During isothermal heating at 845 K, metastable Zr3Al2 phase is transformed into stable Zr2Al3 and ZrAl intermetallic compounds.
References
Y. Sun, Y. Huang, H. Fan, Y. Wang, Z. Ning, F. Liu, D. Feng, X. **, J. Shen, J. Sun, and J.J.J. Chen: J. Non-cryst. Solids, 2015, vol. 419, pp. 82–91.
Z. Zhang, V. Keppens, P.K. Liaw, Y. Yokoyama, and A. Inoue: J. Mater. Res., 2007, vol. 22, pp. 364–67.
T. Wada, T. Zhang, and A. Inoue: Mater. Trans., 2003, vol. 44, pp. 1839–44.
M.L. Morrison, R.A. Buchanan, R.V. Leon, C.T. Liu, B.A. Green, P.K. Liaw, and J.A. Horton: J. Biomed. Mater. Res. A, 2005, vol. 74, pp. 430–38.
Z. Kovács, E. Schafler, P. Szommer, and Á. Révész: J. Alloys Compd., 2014, vol. 593, pp. 207–12.
N.D. de Campos Neto, R.F. de Carvalho Lopes Rocha, F.S. Pereira, C. Soares, F.H. Santa Maria, and M.F. de Oliveira: Metall. Mater. Trans. A, 2020, vol. 51A, pp. 3518–25.
K. ** and J.F. Löffler: Appl. Phys. Lett., 2005, vol. 86, p. 241909.
Q.S. Zhang, W. Zhang, and A. Inoue: Scripta Mater., 2009, vol. 61, pp. 241–44.
Y. Prabhu, A.K. Srivastav, D.V. Gunderov, and J. Bhatt: Physica B, 2022, vol. 624, p. 413416.
A.Y. Churyumov, A.I. Bazlov, A.A. Tsarkov, A.N. Solonin, and D.V. Louzguine-Luzgin: J. Alloys Compd., 2016, vol. 654, pp. 87–94.
C. Ebner, B. Escher, C. Gammer, J. Eckert, S. Pauly, and C. Rentenberger: Acta Mater., 2018, vol. 160, pp. 147–57.
C.A. Pampillo: J. Mater. Sci., 1975, vol. 10, pp. 1194–1227.
E. Ma and J. Ding: Mater. Today, 2016, vol. 19, pp. 568–79.
Y. Yokoyama, K. Yamano, K. Fukaura, H. Sunada, and A. Inoue: Mater. Trans., 2001, vol. 42, pp. 623–32.
M.H. Lee, K.S. Lee, J. Das, J. Thomas, U. Kühn, and J. Eckert: Scripta Mater., 2010, vol. 62, pp. 678–81.
H.B. Yu, J. Hu, X.X. **a, B.A. Sun, X.X. Li, W.H. Wang, and H.Y. Bai: Scripta Mater., 2009, vol. 61, pp. 640–43.
R.Z. Valiev, A.P. Zhilyaev, and T.G. Langdon: Bulk Nanostructured Materials: Fundamentals and Applications, Wiley, Hoboken, NJ, 2014.
Y. Dong, S. Liu, J. Biskupek, Q. Cao, X. Wang, J.Z. Jiang, R. Wunderlich, and H.J. Fecht: Materials (Basel), 2019, https://doi.org/10.3390/ma12101611.
A.M. Glezer, D.V. Louzguine-Luzgin, I.A. Khriplivets, R.V. Sundeev, D.V. Gunderov, A.I. Bazlov, and Y.S. Pogozhev: Mater. Lett., 2019, vol. 256, p. 126631.
D.V. Gunderov, A.A. Churakova, V.V. Astanin, R.N. Asfandiyarov, H. Hahn, and R.Z. Valiev: Mater. Lett., 2020, vol. 261, p. 127000.
A. Jain, Y. Prabhu, D. Gunderov, E.V. Ubyivovk, and J. Bhatt: J. Non-cryst. Solids, 2021, vol. 566, p. 120877.
E.V. Boltynjuk, D.V. Gunderov, E.V. Ubyivovk, M.A. Monclús, L.W. Yang, J.M. Molina-Aldareguia, A.I. Tyurin, A.R. Kilmametov, A.A. Churakova, A.Y. Churyumov, and R.Z. Valiev: J. Alloys Compd., 2018, vol. 747, pp. 595–602.
K.F. Kelton, T.K. Croat, A.K. Gangopadhyay, L.Q. **ng, A.L. Greer, M. Weyland, X. Li, and K. Rajan: J. Non-cryst. Solids, 2003, vol. 317, pp. 71–77.
A.L. Greer: Science (80), 1995, vol. 267, pp. 1947–53.
D. Gunderov and V. Astanin: Metals, 2020, https://doi.org/10.3390/met10030415.
R.Z. Valiev, D.V. Gunderov, A.P. Zhilyaev, A.G. Popov, and V.G. Pushin: J. Metastable Nanocryst. Mater., 2004, vol. 22, pp. 21–26.
Z. Kovács, P. Henits, A.P. Zhilyaev, and Á. Révész: Scripta Mater., 2006, vol. 54, pp. 1733–37.
G. Abrosimova and A. Aronin: J. Alloys Compd., 2018, vol. 747, pp. 26–30.
J.C. Qiao and J.M. Pelletier: J. Non-cryst. Solids, 2011, vol. 357, pp. 2590–94.
D. Roy and H. Raghuvanshi: J. Non-cryst. Solids, 2011, vol. 357, pp. 1701–04.
L.H. Kong, Y.L. Gao, T.T. Song, G. Wang, and Q.J. Zhai: Thermochim. Acta, 2011, vol. 522, pp. 166–72.
S.H. Al-Heniti: J. Alloys Compd., 2009, vol. 484, pp. 177–84.
J.C. Qiao and J.M. Pelletier: Trans. Nonferr. Met. Soc. China (Engl. Ed.), 2012, vol. 22, pp. 577–84.
R. Rashidi, M. Malekan, and R. Gholamipour: J. Non-cryst. Solids, 2018, vol. 498, pp. 272–80.
Y. Luo, H. Ke, R. Zeng, X. Liu, J. Luo, and P. Zhang: J. Non-cryst. Solids, 2020, vol. 528, p. 119728.
Y. Luo, Y. Jiang, P. Zhang, X. Wang, H. Ke, and P. Zhang: Chin. J. Mech. Eng., 2020, vol. 33, pp. 65–71.
C. Peng, Z.H. Chen, X.Y. Zhao, A.L. Zhang, L.K. Zhang, and D. Chen: J. Non-cryst. Solids, 2014, vol. 405, pp. 7–11.
M. Klinger: J. Appl. Crystallogr., 2017, vol. 50, pp. 1226–34.
A. Khond, D.A. Babu, S. Smaran, A. Deshmukh, B. Majumdar, J. Bhatt, and A.K. Srivastav: J. Non-cryst. Solids, 2018, vol. 500, pp. 191–95.
S.V. Madge, A. Caron, R. Gralla, G. Wilde, and S.K. Mishra: Intermetallics, 2014, vol. 47, pp. 6–10.
P. Thompson, D.E. Cox, and J.B. Hastings: J. Appl. Crystallogr., 1987, vol. 20, pp. 79–83.
D.V. Gunderov, E.V. Boltynjuk, V.D. Sitdikov, G.E. Abrosimova, A.A. Churakova, A.R. Kilmametov, and R.Z. Valiev: J. Phys. Conf. Ser., 2018, https://doi.org/10.1088/1742-6596/1134/1/012010.
G. Abrosimova and A. Aronin: Rev. Adv. Mater. Sci., 2017, vol. 50, pp. 55–61.
E.S. Park: Appl. Microsc., 2015, vol. 45, pp. 63–73.
J. Ding, S. Patinet, M.L. Falk, Y. Cheng, and E. Ma: Proc. Natl Acad. Sci. USA, 2014, vol. 111, pp. 14052–56.
C. Fan, M. Imafuku, H. Kurokawa, A. Inoue, and V. Haas: Appl. Phys. Lett., 2001, vol. 79, pp. 1792–94.
R. Babilas, D. Łukowiec, and L. Temleitner: Beilstein J. Nanotechnol., 2017, vol. 8, pp. 1174–82.
F. Xu, Y. Du, P. Gao, Z. Han, and G. Chen: Mater. Lett., 2007, vol. 61, pp. 4875–78.
Y. Zhang, K. Hono, A. Inoue, A. Makino, and T. Sakurai: Acta Mater., 1996, vol. 44, pp. 1497–1510.
C. Fan, D.V. Louzguine, C. Li, and A. Inoue: Appl. Phys. Lett., 1999, vol. 75, pp. 340–42.
A.L. Greer: Acta Metall., 1982, vol. 30, pp. 171–92.
C.A. Schuh, T.C. Hufnagel, and U. Ramamurty: Acta Mater., 2007, vol. 55, pp. 4067–4109.
D.V. Louzguine-Luzgin, G. **e, Q. Zhang, and A. Inoue: Philos. Mag., 2010, vol. 90, pp. 1955–68.
I. Kalay, M.J. Kramer, and R.E. Napolitano: Metall. Mater. Trans. A, 2015, vol. 46A, pp. 3356–64.
R. Rashidi, M. Malekan, and R. Gholamipour: Mater. Sci. Eng. A, 2018, vol. 729, pp. 433–38.
A. Takeuchi and A. Inoue: Mater. Trans., 2005, vol. 46, pp. 2817–29.
S.Z. Zhao, J.H. Li, and B.X. Liu: J. Phys. Condens. Matter, 2013, vol. 25, pp. 1–9.
Prepared under the direction of the A.I.H. Committee: Alloy Phase Diagrams, ASM International, Materials Park, 2016.
Z. Wang, S.V. Ketov, C.L. Chen, Y. Shen, Y. Ikuhara, A.A. Tsarkov, D.V. Louzguine-Luzgin, and J.H. Perepezko: Acta Mater., 2017, vol. 132, pp. 298–306.
D.V. Louzguine-Luzgin, C.L. Chen, L.Y. Lin, Z.C. Wang, S.V. Ketov, M.J. Miyama, A.S. Trifonov, A.V. Lubenchenko, and Y. Ikuhara: Acta Mater., 2015, vol. 97, pp. 282–90.
Y. **ng, Y. Li, X. Wang, X. Yu, T. Zhang, and K. Georgarakis: J. Non-cryst. Solids, 2016, vol. 452, pp. 30–34.
H.E. Kissinger: Anal. Chem., 1957, vol. 29, pp. 1702–06.
J. Málek: Thermochim. Acta, 1995, vol. 267, pp. 61–73.
E. Illekovfi, C. Katarina, F. Kuhnast, and J. Fiorani: Mater. Sci. Eng. A, 1996, vol. 205, pp. 166–79.
W.J. Christian: The Theory of Transformations in Metals and Alloys, Pergamon, Oxford, 2002.
Acknowledgments
One of the authors (Jatin Bhatt) would like to acknowledge the Department of Science and Technology Project Grant (INT/RUS/RFBR/355), (CRG/2019/003674) funded by Government of India for research support. D. V. Gunderov would like to acknowledge the Grant RFBR Research Project 20-08-00497 for funding this work and For XRD characterization of Department of Chemistry, VNIT, Nagpur supported by DST FIST Project No. SR/FST/ CSI-279/2016(C).
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Prabhu, Y., Srivastav, A.K., Churakova, A. et al. Crystallization Kinetics on Melt Spun and HPT-Processed Zr62Cu22Al10Fe5Dy1 Metallic Glass. Metall Mater Trans A 54, 39–52 (2023). https://doi.org/10.1007/s11661-022-06889-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-022-06889-7