Abstract
Purpose
The purpose of this study was to identify the magnetic resonance imaging (MRI) features of uterine endometrial carcinoma (EC) with DNA mismatch repair (MMR) deficiency.
Materials and methods
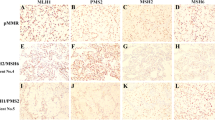
This was a retrospective study approved by our institutional review board. The study included 118 patients pathologically diagnosed as having EC in our institution from April 2014 to December 2016. Of 118 patients, 8 were excluded because of insufficient data. Immunohistochemical analysis of MMR was performed retrospectively to observe the expressions of MLH1, MSH2, MSH6, and PMS2. A tumor with MMR deficiency was detected in 17 of 110 cases (15%). Clinical background characteristics and MRI findings were reviewed. These findings were compared between MMR deficiency group and the other group as a control group. Statistical significance was determined using the Fisher’s exact test and the Mann–Whitney U test, as appropriate.
Results
The clinical background characteristics of patients with EC with MMR deficiency were not significantly different from those of other patients. On MRI, the tumor was significantly more often located in the lower uterine site (MMR(−) vs. MMR(+): 29.4 vs. 8.9% [p = 0.0366]).
Conclusion
EC with MMR deficiency tends to be located lower in the uterus, though most other findings were not significantly different from those of EC without MMR deficiency.


Similar content being viewed by others
Abbreviations
- MMR:
-
Mismatch repair
- MSI:
-
Microsatellite instability
- ADC:
-
Apparent diffusion coefficient
- CRC:
-
Colorectal cancer
- DWI:
-
Diffusion-weighted imaging
- IHC:
-
Immunohistochemistry
- MRI:
-
Magnetic resonance imaging
- TMA:
-
Tissue microarray
- EC:
-
Endometrial carcinoma
- T1WI:
-
T1-weighted imaging
- T2WI:
-
T2-weighted imaging
- CE-T1WI:
-
Contrast-enhanced T1-weighted imaging
- PTE:
-
Peritumoral enhancement
- SEE:
-
Subendometrial enhancement
- LUS:
-
Lower uterine segment
- MSI:
-
Microsatellite instability
References
Hampel H, Frankel W, Panescu J, Lockman J, Sotamaa K, Fix D, et al. Screening for Lynch syndrome (hereditary nonpolyposis colorectal cancer) among endometrial cancer patients. Can Res. 2006;66:7810–7.
Mills AM, Liou S, Ford JM, Berek JS, Pai RK, Longacre TA. Lynch syndrome screening should be considered for all patients with newly diagnosed endometrial cancer. Am J Surg Pathol. 2014;38:1501–9.
Moline J, Mahdi H, Yang B, Biscotti C, Roma AA, Heald B, et al. Implementation of tumor testing for lynch syndrome in endometrial cancers at a large academic medical center. Gynecol Oncol. 2013;130:121–6.
Lynch HT, de la Chapelle A, Hampel H, Wagner A, Fodde R, Lynch JF, et al. American founder mutation for Lynch syndrome. Prevalence estimates and implications. Cancer. 2006;106:448–52.
Lynch HT, Snyder CL, Shaw TG, Heinen CD, Hitchins MP. Milestones of Lynch syndrome: 1895–2015. Nat Rev Cancer. 2015;15:181–94.
Bonadona V, Bonaiti B, Olschwang S, Grandjouan S, Huiart L, Longy M, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA. 2011;305:2304–10.
Stoffel E, Mukherjee B, Raymond VM, Tayob N, Kastrinos F, Sparr J, et al. Calculation of risk of colorectal and endometrial cancer among patients with Lynch syndrome. Gastroenterology. 2009;137:1621–7.
Watkins JC, Yang EJ, Muto MG, Feltmate CM, Berkowitz RS, Horowitz NS, et al. Universal screening for mismatch-repair deficiency in endometrial cancers to identify patients with Lynch syndrome and Lynch-like syndrome. Int J Gynecol Pathol. 2017;36:115–27.
Lu KH, Dinh M, Kohlmann W, Watson P, Green J, Syngal S, et al. Gynecologic cancer as a “sentinel cancer” for women with hereditary nonpolyposis colorectal cancer syndrome. Obstet Gynecol. 2005;105:569–74.
Bhosale P, Ramalingam P, Ma J, Iyer R, Soliman P, Frumovitz M, et al. Can reduced field-of-view diffusion sequence help assess microsatellite instability in FIGO stage 1 endometrial cancer? Reduced FOV diffusion sequence. J Magn Reson Imaging. 2017;45:1216–24.
Fujii S, Kido A, Baba T, Fujimoto K, Daido S, Matsumura N, et al. Subendometrial enhancement and peritumoral enhancement for assessing endometrial cancer on dynamic contrast enhanced MR imaging. Eur J Radiol. 2015;84:581–9.
Moreira L, Balaguer F, Lindor N, de la Chapelle A, Hampel H, Aaltonen LA, et al. Identification of Lynch syndrome among patients with colorectal cancer. JAMA. 2012;308:1555–65.
Ollikainen M, Abdel-Rahman WM, Moisio AL, Lindroos A, Kariola R, Jarvela I, et al. Molecular analysis of familial endometrial carcinoma: a manifestation of hereditary nonpolyposis colorectal cancer or a separate syndrome? J Clin Oncol. 2005;23:4609–16.
Ferguson SE, Aronson M, Pollett A, Eiriksson LR, Oza AM, Gallinger S, et al. Performance characteristics of screening strategies for Lynch syndrome in unselected women with newly diagnosed endometrial cancer who have undergone universal germline mutation testing. Cancer. 2014;120:3932–9.
Mills AM, Sloan EA, Thomas M, Modesitt SC, Stoler MH, Atkins KA, et al. Clinicopathologic comparison of Lynch syndrome-associated and “Lynch-like” endometrial carcinomas identified on universal screening using mismatch repair protein immunohistochemistry. Am J Surg Pathol. 2016;40:155–65.
Haraldsdottir S, Hampel H, Tomsic J, Frankel WL, Pearlman R, de la Chapelle A, et al. Colon and endometrial cancers with mismatch repair deficiency can arise from somatic, rather than germline, mutations. Gastroenterology. 2014;147:1308–16.
Wong A, Ngeow J. Hereditary syndromes manifesting as endometrial carcinoma: How can pathological features aid risk assessment? Biomed Res Int. 2015;2015:1–17.
Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Ruschoff J, et al. Revised Bethesda guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96:261–8.
Vasen HF, Watson P, Mecklin JP, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology. 1999;116:1453–6.
Sjursen W, Haukanes BI, Grindedal EM, Aarset H, Stormorken A, Engebretsen LF, et al. Current clinical criteria for Lynch syndrome are not sensitive enough to identify MSH6 mutation carriers. J Med Genet. 2010;47:579–85.
Terdiman JP. It is time to get serious about diagnosing Lynch syndrome (hereditary nonpolyposis colorectal cancer with defective DNA mismatch repair) in the general population. Gastroenterology. 2005;129:741–4.
Mas-Moya J, Dudley B, Brand RE, Thull D, Bahary N, Nikiforova MN, et al. Clinicopathological comparison of colorectal and endometrial carcinomas in patients with Lynch-like syndrome versus patients with Lynch syndrome. Hum Pathol. 2015;46:1616–25.
Carethers JM. Differentiating Lynch-like from Lynch syndrome. Gastroenterology. 2014;146:602–4.
Rodriguez-Soler M, Perez-Carbonell L, Guarinos C, Zapater P, Castillejo A, Barbera VM, et al. Risk of cancer in cases of suspected Lynch syndrome without germline mutation. Gastroenterology. 2013;144:926–32 e1 (quiz e13–4).
Win AK, Buchanan DD, Rosty C, MacInnis RJ, Dowty JG, Dite GS, et al. Role of tumour molecular and pathology features to estimate colorectal cancer risk for first-degree relatives. Gut. 2015;64:101–10.
Rothan SM, Menias CO, ElGuindy YM, Jensen CT, Shaaban AM, Bhosale P, et al. Imaging of hereditary tumors of the female genital system. J Comput Assist Tomogr. 2017;41:364–75.
Minamiguchi K, Takahama J, Marugami N, Marugami A, Haga M, Takewa M, et al. MR findings of Lynch syndrome-related uterine endometrial carcinoma: a case report. Abdom Radiol. 2016;41:1703–6.
Matoba Y, Kisu I, Saotome K, Katayama M, Taniguchi M, Miura Y, et al. Clear cell carcinoma of the lower uterine segment: a case report. Mol Clin Oncol. 2016;5:701–4.
Westin SN, Lacour RA, Urbauer DL, Luthra R, Bodurka DC, Lu KH, et al. Carcinoma of the lower uterine segment: a newly described association with Lynch syndrome. J Clin Oncol. 2008;26:5965–71.
Sorvari TE, Laakso L. Histochemical investigation of epithelial mucosubstances in the uterine isthmus. Obstet Gynecol. 1970;36:76–81.
Shia J. Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. J Mol Diagn. 2008;10:293–300.
Ryan P, Mulligan AM, Aronson M, Ferguson SE, Bapat B, Semotiuk K, et al. Comparison of clinical schemas and morphologic features in predicting Lynch syndrome in mutation-positive patients with endometrial cancer encountered in the context of familial gastrointestinal cancer registries. Cancer. 2012;118:681–8.
Walsh MD, Cummings MC, Buchanan DD, Dambacher WM, Arnold S, McKeone D, et al. Molecular, pathologic, and clinical features of early-onset endometrial cancer: identifying presumptive Lynch syndrome patients. Clin Cancer Res. 2008;14:1692–700.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Kiyoyuki Minamiguchi declares that he has no conflict of interest. Junko Takahama declares that she has no conflict of interest. Tomoko Uchiyama declares that she has no conflict of interest. Ryosuke Taiji declares that he has no conflict of interest. Natsuhiko Saito declares that he has no conflict of interest. Hiroshi Okada declares that he has no conflict of interest. Nagaaki Marugami declares that he has no conflict of interest. Yasuhito Tanase declares that he has no conflict of interest. Ryuji Kawaguchi declares that he has no conflict of interest. Chiho Ohbayashi declares that she has no conflict of interest. Hiroshi Kobayashi declares that he has no conflict of interest. Toshiko Hirai declares that she has no conflict of interest. Kimihiko Kichikawa declares that he has no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
About this article
Cite this article
Minamiguchi, K., Takahama, J., Uchiyama, T. et al. Uterine endometrial carcinoma with DNA mismatch repair deficiency: magnetic resonance imaging findings and clinical features. Jpn J Radiol 36, 429–436 (2018). https://doi.org/10.1007/s11604-018-0741-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-018-0741-4