Summary
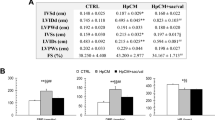
To investigate the molecular mechanism by which Tanshinone IIA (TSN IIA) prevents left ventricular hypertrophy (LVH), we examined the expression of AT1R, TGF-β1 and Smads gene in the hypertrophic myocardium of hypertensive rats with abdominal aorta constriction. LVH model was established by creating abdominal aorta constriction. Four weeks later, animals were randomly divided into 4 groups with 8 animals in each. One group was used as model control, the other three groups were treated with TSN IIA (20 mg/kg), TSN IIA (10 mg/kg) and valsartan (10 mg/kg), respectively. Another 8 SD rats were subjected to sham surgery and served as blank control. After 8-week treatment, the caudal artery pressure of the animals was measured. The tissues of left ventricle were taken for the measurement of the left ventricular mass index (LVMI) and pathological sectioning and HE-staining were used for determining the myocardial fiber dimension (MFD). The mRNA expression of AT1R, protein expression of TGF-beta1 and activity of Smad-2, 4, 7 were detected by RT-PCR and Western blotting, respectively. Our results showed that (1) the blood pressure of rats treated with TSN IIA, either at high or low dose, was significantly higher than those in the control and valsartan-treated group (P<0.01, P<0.05); (2) LVMI and MFD in TSN IIA and valsartan-treated rats were higher than those in the control group (P<0.05) but significantly lower than those in the model control (P<0.01); (3) the high doses of TSN IIA and valsartan significantly down-regulated the mRNA expression of AT1R and protein expression of TGF-beta1 and Smad-3 in the hypertrophic myocardium (P<0.01), and TGF-beta1 in valsartan-treated animals was more significantly lower than that in rats treated with TSN IIA; (4) the two doses of TSN IIA and valsartan significantly up-regulated the protein expression of Smad-7 in the hypertrophic myocardium (P<0.01), and Smad-7 in the animals treated with high-dose TSN IIA was significantly higher than that in rats treated with valsartan. It is concluded that inhibition of myocardial hypertrophy induced by TSN IIA independent of blood pressure. The underlying mechanism might be the down-regulated expression of AT1R mRNA and Smad-3, increased production of Smad-7, and blocking effect of TSN IIA on TGF beta1/Smads signal pathway in local myocardium.
Similar content being viewed by others
References
Carreño JE, Apablaza F, María Paz Ocaranza, et al. Cardiac hypertrophy: molecular and cellular events. Rev Esp Cardiol, 2006,59(5):473–486
Yasunari K, Maeda K, Nakamura M, et al. Left ventricular hypertrophy and angiotensin II receptor blocking agents. Curr Med Chem Cardiovasc Hematol Agents, 2005,3(1): 61–67
Kosugi R, Shioi T, Watanabe-Maeda K, et al. Angiotensin II receptor antagonist attenuates expression of aging markers in diabetic mouse heart. Circ, 2006,70(4):482–488
Schultz Jel J, Witt SA, Glascock BJ, et al. TGF-beta 1 mediates the hypertrophic cardiomyocyte growth induced by angiotensin II. J Clin Invest, 2002,109(6):787–795
Hao J, Wang B, Jones SC, et al. Interaction between angiotensin II and Smad proteins in fibroblasts in failing heart and in vitro. Am J Physiol Heart Circ Physiol, 2000,279(6):3020–3030
Li YS, Wang ZH, Yan L, et al. Effect of tashinone on nitric oxide synthase in hypertrophic cadiocyte of rats suffered abdominal aorta constriction. Zhongguo Zhong Yao Za Zhi (Chinese), 2008,33(12):1446–1450
Wang ZH, Liang QS, Zheng Z. The effect of tanshinone on L-type calcium current in hypertrophic myocardium. Chin J Hypertension (Chinese), 2006,14(6):450–454.
Zhan CY, Tao XL, Han SJ, et al. Effects of tanshinone II A on the cardiac content of aldosterone and the expression of CYP11B1 and CYP11B2 mRNA related to aldosterone synthesis in spontaneously hypertensive rats. Chin Pharm J (Chinese), 2005,40(1):31–33
Rose M, Balakumar P, Singh M, et al. Ameliorative effect of combination of fenofibrate and rosiglitazone in pressure overload-induced cardiac hypertrophy in rats. Pharmacology, 2007,80(2–3):177–184
Zhou D, Liang Q, He X, et al. Changes of c-fos and c-jun mRNA expression in angiotensin II-induced cardiomyocyte hypertrophy and effects of sodium tanshinone IIA sulfonate. J Huazhong Univ Sci Technol [Med Sci], 2008,28(5):531–534
Ruiz-Ortega M, Rodríguez-Vita J, Sanchez-Lopez E, et al. TGF-beta signaling in vascular fibrosis. Cardiovasc Res, 2007,74(2):196–206
Chen LL, Yin H, Huang J. Inhibition of TGF-beta1 signaling by eNOS gene transfer improves ventricular remodeling after myocardial infarction through angiogenesis and reduction of apoptosis. Cardiovasc Pathol, 2007,16(4): 221–230
Rosenkranz S, Flesch M, Bohm M, et al. Alterations of beta-adrenergic signaling and cardiac hypertrophy in transgenic mice overexpressing TGF-beta (1).Am J Physiol Heart Circ Physiol, 2002,283(3):1253–1262
Zhang RY, Wang LF, Zhang L, et al. Effects of angiotensin converting enzyme inhibitor, angiotensin II type I receptor blocker and their combination on postinfarcted ventricular remodeling in rats. Chin Med J (Engl), 2006,119(8):649–655
Chuva de Sousa Lopes SM, Feijen A, Korving J, et al. Connective tissue growth factor expression and Smad signaling during mouse heart development and myocardial infarction. Dev Dyn, 2004,231(3):542–550
Brand T, Schneider MD. The TGF-β superfamily in myocardium:ligands, receptors, transduction, and function. J Mol Cell Cardiol,1995,27(1):5–18
Shi Y, Massague J. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell, 2003,113(6):685–700.
Carl-Henrik H, Kohei M, Peter T, et al. TGF-β1 signalling from cell membrance to nucleus through SMAD proteins. Nature, 1997,390(4):465–471
**ao H, Zhang YY. Understanding the role of transforming growth factor-beta signalling in the heart: overview of studies using genetic mouse models. Clin Exp Pharmacol Physiol, 2008,35(3):335–341
Dong C, Li Z, Alvarez R, et al. Microtubule binding to SMADs may regulate TGF-beta activity. Mol Cell, 2000,5(1):27–34
Pokharel S, van Geel PP, Sharma UC, et al. Increased myocardial collagen content in transgenic rats overexpressing cardiac angiotensin-converting enzyme is related to enhanced breakdown of N-acetyl-Ser-Asp-Lys-Pro and increased phosphorylation of Smad2/3. Circulation, 2004, 110(19):3129–3135
Wang B, Hao J, Jones SC, et al. Decreased Smad 7 expression contributes to cardiac fibrosis in the infarcted rat heart. Am J Physiol Heart Circ Physiol, 2002,282(5): H1685–H1696
Huang J, QIN GH, HU CX, et al. Effects of transforming growth factor-beta1 and signal protein Smad3 on rat cardiomyocyte hypertrophy. Chin Med J (Engl), 2004, 117(10):1471–1475
Zhai Y, Gao X, Wu Q, et al. Fluvastatin decreases cardiac fibrosis possibly through regulation of TGF-beta(1)/Smad 7 expression in the spontaneously hypertensive rats. Eur J Pharmacol, 2008,587(1–3):196–203
Itoh S, ten Dijke P. Negative regulation of TGF-β receptor/ Smad signal transduction. Curr Opin Cell Biol, 2007,19(2):176–184
Song L, Yan W, Chen X, et al. Myocardial smad4 is essential for cardiogenesis in mouse embryos. Circ Res, 2007,101(3):277–285
Tang J, Zhan C, Zhou J. Effects of tanshinone IIA on transforming growth factor beta1-Smads signal pathway in renal interstitial fibroblasts of rats. J Huazhong Univ Sci Technolog [Med Sci], 2008,28(5):539–542
Li YS, Wang ZH, Wang J, et al. The effect and molecular mechanism of Tashinon IIA on the hypertrophic cadiocyte due to abdominal aorta stenosis in rats. Chin Pharm J (Chinese), 2008,43(17):1313–1317
Author information
Authors and Affiliations
Corresponding author
Additional information
This project was supported by a grant from the National Natural Science Foundation of China (No. 30500657).
Rights and permissions
About this article
Cite this article
Li, Y., Yang, Y., Yu, D. et al. The Effect of Tanshinone IIA upon the TGF-beta1/Smads signaling pathway in hypertrophic myocardium of hypertensive rats. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 29, 476–480 (2009). https://doi.org/10.1007/s11596-009-0417-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-009-0417-5