Abstract
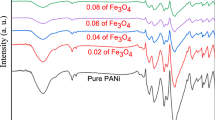
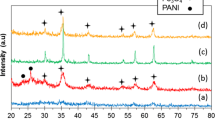
By using inorganic Fe3O4 nanoparticles of different content as nucleation sites, PAn-Fe3O4 nanorods were successfully synthesized through a simple, conventional, and inexpensive one-step in-situ polymerization method. The TEM images revealed the size and morphology of the resultant nanocomposite. The EDS pattern confirmed the existence of Fe3O4 in the composite. The FT-IR spectral analysis confirmed the formation of PAn encapsulated Fe3O4 nanocomposite. With the content of Fe3O4 increasing, the conductivity of the nanocomposites gradually decreases, meanwhile, the saturation magnetization increases and reveals a super paramagnetic behavior. With controllable electrical, magnetic, and electromagnetic properties, the well-prepared nanocomposites may have the potential applications in chemical sensors, catalysis, microwave absorbing, and electro-magneto-rheological fluids, etc.
Similar content being viewed by others
References
S Miyauchi, H Abiko, Y Sorimachi, et al. Preparation of Barium Titanate-polypyrrole Compositions and Their Electrical Properties[J]. J. Appl. Polym. Sci., 1989, 37: 289–293
J G Guan, W Wang, R Z Gong, et al. One-Step Synthesis of Cobalt-Phthalocyanine/Iron Nanocomposite Particles with High Magnetic Susceptibility[J]. Langmuir, 2002, 18: 4 198–4 204
H S Kim, B H Sohn, W Lee, et al. Multifunctional Layer-by-layer Self-assembly of Conducting Polymers and Magnetic Nanoparticles[J]. Thin Solid Film, 2002, 419: 173–177
P Anilkumar, M Jayakannan. New Renewable Resource Amphiphilic Molecular Design for Size-Controlled and Highly Ordered Polyaniline Nanofibers[J]. Langmuir, 2006, 22: 5 952–5 957
X Lu, H Mao, D Chao, et al. Ultrasonic Synthesis of Polyaniline Nanotubes Containing Fe3O4 Nanoparticles[J]. J. Solid State Chem., 2006, 179: 2 609–2 615
C T Chen, Y C Chen. Fe3O4/TiO2 Core/Shell Nanoparticles as Affinity Probes for the Analysis of Phosphopeptides Using TiO2 Surface-Assisted Laser Desorption/Ionization Mass Spectrometry[J]. Anal. Chem., 2005, 77: 5 912–5 219
JCPDS Powder Diffraction File. International Center for Diffraction Data[DB]. Newtown Square, PA, 1980
D O Smith. Magnetization of a Magnetite Single Crystal Near the Curie Point[J]. Phys. Rev., 1956, 102: 959–963
B M Altura, A Gebrewold, A Zhang, et al. Preparation of Nanocrystalline Fe3O4 by γ-ray Radiation[J]. Mater. Lett., 1997, 33: 113–116
Yavuz, M K Ram, M Aldissi, et al. Synthesis and the Physical Properties of MnZn Ferrite and NiMnZn Ferrite-polyaniline Nanocomposite Particles[J]. J. Mater. Chem., 2005, 15: 810–817
W Luzny, E Banka. Relations Between the Structure and Electric Conductivity of Polyaniline Protonated with Camphorsulfonic Acid[J]. Macromolecules, 2000, 33: 425–429
Y **a, J M Wiesinger, A G Macdiarmid. Camphorsulfonic Acid Fully Doped Polyaniline Emeraldine Salt: Conformations in Different Solvents Studied by an Ultraviolet/visible/near-infrared Spectroscopic Method[J]. Chem. Mater., 1995, 7: 443–445
A B Diaz, N D S Mohallem, R D Sinisterra. Preparation of a Ferrofluid Using Cyclodextrin and Magnetite[J]. J. Magn. Magn. Mater. R., 2004, 272: 2 395–2 397
A G MacDiamid, J C Chiang, M Halpern, et al. “Polyaniline”: Interconversion of Metallic and Insulating Forms[J]. Cryst. Liq. Cryst., 1985, 121: 173–180
S Wei, Y Zhu, Y Zhang, et al. Preparation and Characterization of Hyperbranched Aromatic Polyamides/Fe3O4 Magnetic Nanocomposite[J]. React. Funct. Polym., 2006, 66: 1 272–1 277
A Bocanegra, N D S Mohallem, R D Sinisterra. Complex Material Using Beta-Cyclodextrins and Nickel-zinc Ferrite to Obtain a Magnetically Targetable Drug Carrier[J]. Mater. Res. Soc. Symp. Proc., 2002, 711: 30–35
H Q **e, J G Guan, J S Guo. Three Ways to Improve Electroheological Properties of Polyaniline-based Suspensions[J]. J. Appl. Poly. Sci., 1997, 64: 1 641–1 647
N Z Kazantseva, J Vilcakova, V Kresalek, et al. Magnetic Behaviour of Composites Containing Polyaniline-coated Manganese-zinc Ferrite[J]. J. Magn. Magn.Mater., 2004, 269: 30–37
B Tang, Y Geng, Q Sun, et al. Processible Nanomaterials with High Conductivity and Magnetizability. Preparation and Properties of Maghemite/polyaniline Nanocomposite Films[J]. Pure Appl. Chem., 2000, 72: 157–162
J Deng, C He, Y Peng, et al. Magnetic and Conductive Fe3O4-polyaniline Nanoparticles with Core-shell Structure[ J]. Synth. Met., 2003, 139: 295–301
D Y Godovsky. Device Applications of Polymer-Nanocomposites[J]. Adv. Polym. Sci., 2000, 153: 163–205
G Bidan, O Jarjayes, Fruchart J M. New Nanocomposites Based on Tailor Dressed Magnetic Particles in a Polypyrrole Matrix[J]. Adv. Mater., 1994, 6: 152–155
M Kryszewski, J K Jeszka. Nanostructured Conducting Polymer Composites-superparamagnetic Particles in Conducting Polymers[J]. Synth. Met., 1998, 94: 99–104
I Sapurina, A Y Osadchev, B Z Volchek. In-situ Polymerized Polyaniline Films: Brush-like Chain Ordering[J]. Synth. Met., 2002, 129: 29–37
M Fahlman, S Jasty, A J Epstein. Corrosion Protection of Iron/steel by Emeraldine Base Polyaniline: an X-ray Photoelectron Spectroscopy Study[J]. Synth. Met., 1997, 85: 1 323–1 326
X D Chen, X M He. The Effect of the Recess Shape on Performance Analysis of the Gas-lubricated Bearing in Optical Lithography[J]. Tribology International, 2006, 39(11): 1 336–1 341
He X M, Chen X D. The Dynamic Analysis of the Gas Lubracated Stage in Optical Lithography[J]. International Journal of Advanced Manufacturing Technology, 2007, 32(9–10): 978–984
Author information
Authors and Affiliations
Corresponding author
Additional information
Funded by National Natural Science Foundation of China(No.10974148), Sub-project of State Key Development Program of Basic Research of China(Nos. 2009CB939704 and 2009CB939705
Rights and permissions
About this article
Cite this article
Leng, C., Wei, J., Liu, Z. et al. Synthesis of polyaniline-Fe3O4 nanocomposites and their conductivity and magnetic properties. J. Wuhan Univ. Technol.-Mat. Sci. Edit. 25, 760–764 (2010). https://doi.org/10.1007/s11595-010-0087-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11595-010-0087-y