Abstract
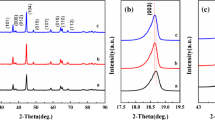
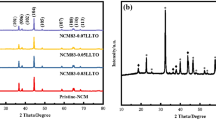
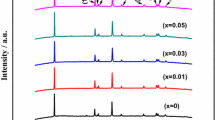
Layered Li-rich cathode materials Li1.2Mn0.534Ni0.133Co0.133O2 (LNCMN-0) and Na do** Li1.1Na0.1Mn0.534Ni0.133Co0.133O2 (LNCMN-0.1) are prepared successfully by a co-precipitation method and several consecutive calcination treatments. Besides, the phase structure, morphology, and electrochemical properties of the four samples are studied in detail using X-ray diffraction (XRD), scanning electron microscope (SEM), galvanostatic charge-discharge test, cyclic voltammetry (CV), and electrochemical impedance spectroscopy (EIS). Although the discharge capacity of spherical LNCMN-0.1 decreases slightly at 0.1 C (1 C = 250 mA g−1), compared to the pristine LNCMN-0, it is noteworthy that the LNCMN-0.1 matched with dual Li+/Na+ electrolyte exhibit superior stability performance at 1 C, as well as enhanced rate capability. The LNCMN-0.1 (Li+/Na+) delivers an initial discharge specific capacity of 267.61 mAh g−1 at 0.1 C between 2.0 and 4.8 V at room temperature and initial coulombic efficiency of 83.51%, which is higher than the LNCMN-0 samples (76.42 and 81.54%). The experimental results verify that Na do** combined with dual Li+/Na+ electrolyte can generate a synergistic effect, which is a promising idea to ameliorate the electrochemical performance for this material.







Similar content being viewed by others
References
Qu LN, Hou XH, Huang XY, Liang Q, Ru Q, Wu B, Lam KH (2017) Self-assembled porous NiFe2O4 floral microspheres inlaid on ultrathin flake graphite as anode materials for Lithium batteries. ChemElectroChem 4:3148–3155. https://doi.org/10.1002/celc.201700862
Huang ZJ, Wang ZX, **g Q, Guo H, Li X, Yang Z (2016) Investigation on the effect of Na do** on structure and Li-ion kinetics of layered LiNi0.6Co0.2Mn0.2O2 cathode material. Electrochim Acta 192:120–126. https://doi.org/10.1016/j.electacta.2016.01.139
Chen HD, Hou XH, Qu LN, Qin H, Ru Q, Huang Y, Hu S, Lam KH (2017) Electrochemical properties of core–shell nano-Si@carbon composites as superior anode materials for high-performance Li-ion batteries. J Mater Sci Mater Electron 28:250–258. https://doi.org/10.1007/s10854-016-5518-x
Xu JT, Ma JM, Fan QH, Guo S, Dou S (2017) Recent progress in the design of advanced cathode materials and battery models for high-performance Lithium-X (X = O2, S, Se, Te, I2, Br2 ) batteries. Adv Mater 29:1–20. https://doi.org/10.1002/adma.201606454
Kolek M, Otteny F, Schmidt P, Mück-Lichtenfeld C, Einholz C, Becking J, Schleicher E, Winter M, Bieker P, Esser B (2017) Ultra-high cycling stability of poly(vinylphenothiazine) as a battery cathode material resulting from π–π interactions. Energy Environ Sci 10:2334–2341. https://doi.org/10.1039/c7ee01473b
Huang YL, Hou XH, Ma SM, Zou X, Wu Y, Hu S, Shao Z, Liu X (2015) Template GNL-assisted synthesis of porous Li1.2Mn0.534Ni0.133Co0.133O2: towards high performance cathodes for lithium ion batteries. RSC Adv 5:25258–25265. https://doi.org/10.1039/c5ra00845j
Yao J, Wang XL, Zhao XR et al (2016) Electrospun Li2MnO3-modified Li1.2NixCo0.1Mn0.9-xO2 nanofibers: synthesis and enhanced electrochemical performance for lithium-ion batteries. Electron Mater Lett 12:804–811. https://doi.org/10.1007/s13391-016-6171-5
Tan SY, Wang L, Bian L, Xu JB, Ren W, Hu PF, Chang AM (2015) Highly enhanced low temperature discharge capacity of LiNi1/3Co1/3Mn1/3O2 with lithium boron oxide glass modification. J Power Sources 277:139–146. https://doi.org/10.1016/j.jpowsour.2014.11.149
Kobayashi G, Irii Y, Matsumoto F, Ito A, Ohsawa Y, Yamamoto S, Cui Y, Son JY, Sato Y (2016) Improving cycling performance of Li-rich layered cathode materials through combination of Al2O3-based surface modification and stepwise precycling. J Power Sources 303:250–256. https://doi.org/10.1016/j.jpowsour.2015.11.014
Liang LW, Du K, Peng ZD et al (2014) Co–precipitation synthesis of Ni0.6Co0.2Mn0.2(OH)2 precursor and characterization of LiNi0.6Co0.2Mn0.2O2 cathode material for secondary lithium batteries. Electrochim Acta 130:82–89. https://doi.org/10.1016/j.electacta.2014.02.100
**a H, Lu L, Meng YS, Ceder G (2007) Phase transitions and high-voltage electrochemical behavior of LiCoO2 thin films grown by pulsed laser deposition. J Electrochem Soc 154:A337–A342. https://doi.org/10.1149/1.2509021
Guo B, Zhao JH, Fan XM, Zhang W, Li S, Yang Z, Chen Z, Zhang W (2017) Aluminum and fluorine co-do** for promotion of stability and safety of lithium-rich layered cathode material. Electrochim Acta 236:171–179. https://doi.org/10.1016/j.electacta.2017.03.133
Liu H, Cao Q, Fu LJ et al (2006) Do** effects of zinc on LiFePO4 cathode material for lithium ion batteries. Electrochem. Commun 8:1553–1557. https://doi.org/10.1016/j.elecom.2006.07.014
Wu CY, Huang W, Liu LF, Wang H, Zeng Y, **e J, ** C, Zhang Z (2016) Facile synthesis of hierarchical β-LiFePO4 and its phase transformation to electrochemically active α-LiFePO4 for Li-ion batteries. CrystEng Comm 18:7707–7714. https://doi.org/10.1039/c6ce01294a
Wang FX, **ao SY, Zhu YS, Chang Z, Hu CL, Wu YP, Holze R (2014) Spinel LiMn2O4 nanohybrid as high capacitance positive electrode material for supercapacitors. J Power Sources 246:19–23. https://doi.org/10.1016/j.jpowsour.2013.07.046
Wang FX, **ao SY, Gao XW, Zhu YS, Zhang HP, Wu YP, Holze R (2013) Nanoporous LiMn2O4 spinel prepared at low temperature as cathode material for aqueous supercapacitors. J Power Sources 242:560–565. https://doi.org/10.1016/j.jpowsour.2013.05.115
Wang SF, Sha YJ, Zhu YL, Xu X, Shao Z (2015) Modified template synthesis and electrochemical performance of a Co3O4/mesoporous cathode for lithium-oxygen batteries. J Mater Chem A 3:16132–16141. https://doi.org/10.1039/C5TA03091A
Wang SF, Suo Y, Su C, Chen Y, Zhu Y, Shao Z (2016) Graphene decorated with multiple nanosized active species as dual function electrocatalysts for lithium-oxygen batteries. Electrochim Acta 188:718–726. https://doi.org/10.1016/j.electacta.2015.12.046
Wang FX, **ao SY, Shi Y, Liu LL, Zhu YS, Wu YP, Wang JZ, Holze R (2013) Spinel LiNixMn2−xO4 as cathode material for aqueous rechargeable lithium batteries. Electrochim Acta 93:301–306. https://doi.org/10.1016/j.electacta.2013.01.106
Tang ZH, Zheng HH, Qian FP, Ma Y, Zhao C, Song L, Chen Y, **ong X, Zhu X, Mi C (2018) Improvement of cycling and thermal stability of LiNi0.8Mn0.1Co0.1O2 cathode material by secondly treating process. Ionics 24:61–71. https://doi.org/10.1007/s11581-017-2179-6
Kasnatscheew J, Evertz M, Streipert B, Wagner R, Klöpsch R, Vortmann B, Hahn H, Nowak S, Amereller M, Gentschev AC, Lamp P, Winter M (2016) The truth about the 1st cycle coulombic efficiency of LiNi1/3Co1/3Mn1/3O2 (NCM) cathodes. Phys Chem Chem Phys 18:3956–3965. https://doi.org/10.1039/c5cp07718d
Liu W, Oh P, Liu X, Lee MJ, Cho W, Chae S, Kim Y, Cho J (2015) Nickel-rich layered lithium transition-metal oxide for high-energy lithium-ion batteries. Angew Chem Int Ed 54:4440–4457. https://doi.org/10.1002/anie.201409262
Yao L, Feng Y, ** GX (2015) A new method for the synthesis of LiNi1/3Co1/3Mn1/3O2 from waste lithium ion batteries. RSC Adv 5:44107–44114. https://doi.org/10.1039/C4RA16390G
Lu C, Wu H, Zhang Y, Liu H, Chen B, Wu N, Wang S (2014) Cerium fluoride coated layered oxide Li1.2Mn0.54Ni0.13Co0.13O2 as cathode materials with improved electrochemical performance for lithium ion batteries. J Power Sources 267:682–691. https://doi.org/10.1016/j.jpowsour.2014.05.122
Hu CL, Yi HH, Wang FX, ** at P-site to improve electrochemical performance of LiMnPO4 as cathode for lithium ion battery. J Power Sources 255:355–359. https://doi.org/10.1016/j.jpowsour.2013.12.040
**e HB, Du K, Hu GR et al (2016) The role of sodium in LiNi0.8Co0.15Al0.05O2 cathode material and its electrochemical behaviors. J Phys Chem C 120:3235–3241. https://doi.org/10.1021/acs.jpcc.5b12407
Jiang YX, Zhou F, Wang CL, Kong J, Xu L (2017) Influence of co-precipitation temperature on microstructure and electrochemical properties of Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathode materials for lithium ion batteries. Ionics 23:585–596. https://doi.org/10.1007/s11581-016-1863-2
Zhao RR, Yang ZL, Liang JX, Lu D, Liang C, Guan X, Gao A, Chen H (2016) Understanding the role of Na-do** on Ni-rich layered oxide LiNi0.5Co0.2Mn0.3O2. J Alloy Compd 689:318–325. https://doi.org/10.1016/j.jallcom.2016.07.230
Chen Z, Wang J, Chao DL, Baikie T, Bai L, Chen S, Zhao Y, Sum TC, Lin J, Shen Z (2016) Hierarchical porous LiNi1/3Co1/3Mn1/3O2 Nano−/micro spherical cathode material: minimized cation mixing and improved Li+ mobility for enhanced electrochemical performance. Sci Rep 6:25771. https://doi.org/10.1038/srep25771
Wang YX, Shang KH, He W, Ai XP, Cao YL, Yang HX (2015) Magnesium-doped Li-1.2[Co0.13Ni0.13Mn0.54]O-2 for Lithium-ion battery cathode with enhanced cycling stability and rate capability. ACS Appl Mater Interfaces 7:13014–13021. https://doi.org/10.1021/acsami.5b03125
Jiang L, Fu CP, Li KQ, Zhou H, Huang Y, Kuang Y (2017) K-doped Li3V2(PO4)3: a novel cathode material for high performance lithium-ion batteries. Mater Lett 198:73–75. https://doi.org/10.1016/j.matlet.2017.04.014
Chen L, Gu QW, Zhou XF et al (2013) New-concept batteries based on aqueous Li+/Na+ mixed-ion electrolytes. Sci. Rep 3:1946. https://doi.org/10.1038/srep01946
Wang JX, Wang ZX, Li XH, Guo H, Wu X, Zhang X, **ao W (2013) xLi3V2(PO4)3·LiVPO4F/C composite cathode materials for lithium ion batteries. Electrochim Acta 87:224–229. https://doi.org/10.1016/j.electacta.2012.09.014
Shi SJ, Tu JP, Mai YJ, Zhang YQ, Gu CD, Wang XL (2012) Effect of carbon coating on electrochemical performance of Li1.048Mn0.381Ni0.286Co0.286O2 cathode material for lithium-ion batteries. Electrochim Acta 63:112–117. https://doi.org/10.1016/j.electacta.2011.12.082
Zheng JM, Wu XBYY (2011) A comparison of preparation method on the electrochemical performance of cathode material Li[Li0.2Mn0.54Ni0.13Co0.13]O2 for lithium ion battery. Electrochim Acta 56:3071–3078. https://doi.org/10.1016/j.electacta.2010.12.049
Kim H-J, ** B-S, Doh C-H, Bae D-S, Kim HS (2013) Improved electrochemical performance of doped-LiNi0.5Mn1.5O4 cathode material for lithium-ion batteries. Electron Mater Lett 9:851–854. https://doi.org/10.1007/s13391-013-6028-0
Funding
This work is financially supported by the union project of National Natural Science Foundation of China and Guangdong Province (No. U1601214), the Scientific and Technological Plan of Guangdong Province (2016B010114002, 2017B090901027), the Scientific and Technological Plan of Guangzhou City (201607010322), the LanDun information security technology open fund (LD20170210), and the Innovation Project of Graduate School of South China Normal University (2017LKXM081).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, Y., Hou, X., Shen, K. et al. Li1.1Na0.1Mn0.534Ni0.133Co0.133O2 as cathode with ameliorated electrochemical performance based on dual Li+/Na+ electrolyte. Ionics 25, 51–59 (2019). https://doi.org/10.1007/s11581-018-2587-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-018-2587-2