Abstract
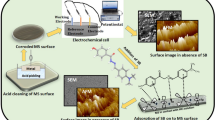
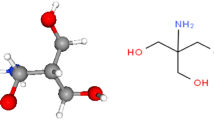
The inhibition efficiency of 2-aminoethanethiol (2-AEE) has been investigated against steel corrosion in 0.1 M HCl solution. The effect of temperature, pH, and concentration were studied with the help of potentiodynamic measurement, electrochemical impedance spectroscopy, scanning electron microscopy, and atomic absorption spectroscopy techniques. The potential of zero charge (Epzc) studies showed that the adsorption occurs via −SH group; the metal surface is positively charged in corrosive test solution. The adsorptive interaction is evaluated, and best correlation was obtained with Langmuir isotherm. 2-AEE was shown to have significant inhibition efficiency against steel corrosion. The response surface methodology was employed to explain the relation between pH, inhibitor concentration, and the efficiency. The regression analysis was realized for development of an equation between independent variables and the output. The success of fitting model was tested with basic statistical arguments, residual and variance analysis, T and F tests, and R 2 value. The statistical evaluations showed that the obtained polynomial equation can be successfully used for optimization of applications involving the use of 2-AEE as inhibitor.











Similar content being viewed by others
References
Bouayed M, Rabaa H, Srhiri A, Saillard JY, Bachir AB, Le Beuze A (1998) Experimental and theoretical study of organic corrosion inhibitors on iron in acidic medium. Corros Sci 41:501–517
Elkadi L, Mernari B, Traisnel M, Bentiss F, Lagrenée M (2000) The inhibition action of 3,6-bis(2-methoxyphenyl)-1,2-dihydro-1,2,4,5-tetrazine on the corrosion of mild steel in acidic media. Corros Sci 42:703–719
Bentiss F, Lebrini M, Lagrenée M (2005) Thermodynamic characterization of metal dissolution and inhibitor adsorption processes in mild steel/2,5-bis(n-thienyl)-1,3,4-thiadiazoles/hydrochloric acid system. Corros Sci 47:2915–2931
Mahgoub FM, Abdel-Nabey BA, El-Samadisy YA (2010) Adopting a multipurpose inhibitor to control corrosion of ferrous alloys in cooling water systems. Mater Chem Phys 120:104–108
Solmaz R, Kardaş G, Çulha M, Yazıcı B, Erbil M (2008) Investigation of adsorption and inhibitive effect of 2-mercaptothiazoline on corrosion of mild steel in hydrochloric acid media. Electrochim Acta 53:5941–5952
Cheng XL, Ma HY, Chen SH, Yu R, Chen X, Yao ZM (1998) Corrosion of stainless steels in acid solutions with organic sulfur-containing compounds. Corros Sci 41:321–333
Mert BD, Mert ME, Kardaş G, Yazıcı B (2011) Experimental and theoretical investigation of 3-amino-1,2,4-triazole-5-thiol as a corrosion inhibitor for carbon steel in HCl medium. Corros Sci 53:4265–4272
Li X, Deng S, Fu H (2012) Allyl thiourea as a corrosion inhibitor for cold rolled steel in H3PO4 solution. Corros Sci 55:280–288
Li W, He Q, Pei C, Hou B (2007) Experimental and theoretical investigation of the adsorption behaviour of new triazole derivatives as inhibitors for mild steel corrosion in acid media. Electrochim Acta 52:6386–6394
Bouayed M, Rabaa H, Srhiri A, Saillard JY, Bachir AB, Le Beuze A (1998) Allyl thiourea as a corrosion inhibitor for cold rolled steel in H3PO4 solution. Corros Sci 41:501–517
Tüken T, Kıcır N, Elalan NT, Sığırcık G, Erbil M (2012) Self assembled film based on hexane-1,6-diamine and 2-mercapto-ethanol on copper. Appl Surf Sci 258:6793–6799
Raja PB, Sethuraman MG (2008) Natural products as corrosion inhibitor for metals in corrosive media—a review. Mater Lett 62:113–116
Negm NA, Kandile NG, Aiad IA, Mohammad MA (2011) New eco-friendly cationic surfactants: synthesis, characterization and applicability as corrosion inhibitors for carbon steel in 1 N HCl. Colloids Surfaces A: Physicochem Eng Aspects 391:224–233
Ghareba S, Omanovic S (2010) Interaction of 12-aminododecanoic acid with a carbon steel surface: towards the development of ‘green’ corrosion inhibitors. Corros Sci 52:2104–2113
Moretti G, Guidi F, Grion G (2004) Tryptamine as a green iron corrosion inhibitor in 0.5 M deaerated sulphuric acid. Corros Sci 46:387–403
Goh KH, Lim TT, Chui PC (2008) Evaluation of the effect of dosage, pH and contact time on high-dose phosphate inhibition for copper corrosion control using response surface methodology (RSM). Corros Sci 50:918–927
Liu F, Lu X, Yang W, Lu J, Zhong H, Chang X, Zhao C (2013) Optimizations of inhibitors compounding and applied conditions in simulated circulating cooling water system. Desalination 313:18–27
Bockris JO’M, Reddy AKN, Gamboa-Aldeco M (2000) Modern Electrochemistry, vol 2A. Kluwer Academic/Plenum Publishers, New York
Tüken T, Demir F, Kıcır N, Sığırcık G, Erbil M (2012) Inhibition effect of 1-ethyl-3-methylimidazolium dicyanamide against steel corrosion. Corros Sci 59:110–118
Kern P, Landolt D (2002) Adsorption of a bromine labeled carboxylic acid corrosion inhibitor on iron measured with EQCM, EIS and XPS. Corros Sci 44:1809–1824
Abiola OK, Oforka NC (2004) Adsorption of (4-amino-2-methyl-5-pyrimidinyl methylthio) acetic acid on mild steel from hydrochloric acid solution (HCl)—Part 1. Mater Chem Phys 83:315–322
Popova A, Sokolova E, Raicheva S, Christov M (2003) AC and DC study of the temperature effect on mild steel corrosion in acid media in the presence of benzimidazole derivatives. Corros Sci 45:33–58
Amin MA, Abd El-Rehim SS, El-Sherbini EEF, Bayoumi RS (2007) The inhibition of low carbon steel corrosion in hydrochloric acid solutions by succinic acid: Part I. Weight loss, polarization, EIS, PZC, EDX and SEM studies. Electrochim Acta 52:3588–3600
Oraon B, Majumdar G, Ghosh B (2006) Application of response surface method for predicting electroless nickel plating. Mater Des 27:1035–1045
Zhao X, Jiang R, Zu Y, Wang Y, Zhao Q, Zu B, Zhao D, Wang M, Sun Z (2012) Process optimization studies of 10-hydroxycamptothecin (HCPT)-loaded folate-conjugated chitosan nanoparticles by SAS-ionic crosslink combination using response surface methodology (RSM). Appl Surf Sci 258:2000–2005
Guo R, Ren X, Ren H (2012) A new method for analysis of the toxicity of organophosphorus pesticide, dimethoate on rotifer based on response surface methodology. J Hazard Mater 237:270–276
Baş D, Boyacı IH (2007) Modeling and optimization I: usability of response surface methodology. J Food Eng 78:836–845
Perfetti G, van der Meer DEC, Wildeboer WJ, Meesters GMH (2012) Investigation on resistance to attrition of coated particles by response surface methodology. Adv Powder Technol 23:64–70
Fontana MG, Greene ND (1967) Corrosion engineering. McGraw-Hill Book Company, New York
Acknowledgment
The authors are grateful for the helpful comments and careful corrections about RSM made by Hakan Karaçizmeli and Meryem Ünal Top from BOSSA Denim Company, Turkey.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tansuğ, G., Tüken, T., Kıcır, N. et al. Investigation of 2-aminoethanethiol as corrosion inhibitor for steel using response surface methodology (RSM). Ionics 20, 287–294 (2014). https://doi.org/10.1007/s11581-013-0966-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-013-0966-2