Abstract
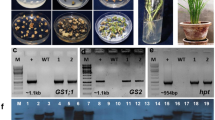
A full-length cDNA that encodes the rice chloroplastic glutamine synthetase 2 gene was isolated from a Minghui 63-normalized cDNA library; and GS2 rice transformants were obtained by an Agrobacterium tumefaciens-mediated transformation method. Transcripts of the GS2 gene were shown to accumulate at higher levels in the primary transgenic plants in the T0 generation; whereas plants in the T1 generation exhibited a co-suppressed chlorosis phenotype (yellow leaves) accompanied by decreased plant height, few tillers and decreased dry weight. The plants with yellow leaves also displayed a significant decline in GS2 messenger RNA (mRNA) transcriptional level and chlorophyll content; a decrease in total GS activities of ˜50% was also found. Although there was no decrease in the concentration of total free amino acids, a change in the concentration of individual amino acids was observed. Our result also indicates a decreased metabolic level (soluble protein content and ammonium concentration) in GS2 co-suppressed plants. A correlation between chlorophyll content and GS2 mRNA expression level was also observed. The GS2 co-suppressed plants showed better performance when complemented with exogenous glutamine, indicating that the lack of an organic nitrogen pool inside the cell is the possible reason for the chlorosis phenotype of the transformants.
Similar content being viewed by others
References
Hirel B, Lea P J. Ammonium Assimilation. In: Lea P J, Morof Gaudry J F, eds. Plant Nitrogen. Berlin: Springer-Verlag, 2001. 79–99
Temple S J, Vance C P, Gantt J S. Glutamate synthase and nitrogen assimilation. Trends Plant Sci, 1998, 3: 51–56
Ireland R J, Lea P J. The enzymes of glutamine, glutamate, asparagines and aspirate metabolism. In: Singh B K, ed. Plant Amino Acids: Biochemistry And Biotechnology. New York: Marcel Dekker, 1999. 49–109
Stewart G R, Mann A F, Fentem P A. Enzymes of glutamate formation: glutamate dehydrogenase, glutamine synthetase and glutamate synthase. In: Miflin B J, ed. The Biochemistry Of Plants: Amino Acids And Derivatives. New York: Academic Press, 1980. 271–327
Zozaya-Garza M, Sengupta-Gopalan C. Glutamine synthetase gene isolation from an alfalfa leaf cDNA library. Plant Physiol, 1999, 119: 1568
Lightfoot D A, Green N K, Cullimore J V. The chloroplast-located glutamine synthetase of Phaseolus vulgaris L.: nucleotide sequence, expression in different organs and uptake into isolated chloroplast. Plant Mol Biol, 1988, 11: 191–202
Tingey S V, Tsai F-Y, Edwards J W, et al. Chloroplast and cytosolic glutamine synthetase are encoded by homologous nuclear genes which are differentially expressed in vivo. J Biol Chem, 1988, 163: 9651–9657
Keegstra K, Cline K. Protein import and routing system of chloroplasts. Plant Cell, 1999, 11: 557–570
May T, Soll J. Chloroplast precursor protein translocon. FEBS Lett, 1999, 452: 53–56
Lam H-M, Coschigano K, Shultz C, et al. Use of Arabidopsis mutants and genes to study amide amino acid biosynthesis. Plant Cell, 1995, 7: 887–898
Kozaki A, Tabeka G. Photorespiration protects C3 plants from photooxidation. Nature, 1996, 384: 557–560
Hoshida H, Tanaka Y, Hibino T, et al. Enhanced tolerance to salt stress in transgenic rice that overexpresses chloroplast glutamine synthetase. Plant Mol Biol, 2000, 43: 103–111
Husted S, Mattsson M, Mollers C, et al. Photorespiratory NH4 + production in leaves of wild-type and glutamine synthetase 2 antisense oilseed rape. Plant Physiol, 2002, 130: 989–998
Chu Z, Peng K, Zhang L, et al. Construction and characterization of a normalized whole-life-cycle cDNA library of rice. Chinese Sci Bull, 2003, 48: 229–235
Maniatis T A, Fritsch E F, Sambrook J. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor: Cold Spring Harbor Laboratory Press, 1992
Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: A laboratory manual, 2nd ed. Cold Spring Harbor: Cold Spring Harbor Laboratory Press, 1989
Hiei Y, Ohta S, Komari T, et al. Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J, 1994, 6: 271–282
Yoshida S, Forno D A, Cook J H, et al. Laboratory manual for physiological studies of rice. 3rd ed. Manila: International Rice Research Institute, 1976
Arnon D I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol, 1949, 24: 1–15
Melo P M, Lima L M, Santos I M, et al. Expression of the plastid-located glutamine synthetase of Medicago truncatula. Accumulation of the precursor in root nodules reveals an in vivo control at the level of protein import into plastids. Plant Physiol, 2003, 132: 390–399
Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein binding. Anal Biochem, 1976, 72: 248–254
O’Neal D, Joy K W. Glutamine synthetase of pea leaves: Purification, stabilization and pH optima. Arch Biochem Biophys, 1973, 159: 113–122
Gordon S A, Fleck A, Bell J. Optimal conditions for the estimation of ammonium by the Berthelot reaction. Ann Clin Biochem, 1978, 15: 270–275
Lancien M, Gadal P, Hodges M. Enzyme redundancy and the importance of 2-oxoglutarate in higher plant ammonium assimilation. Plant Physiol, 2000, 123: 817–824
Wallsgrove R M, Turner J C, Hall N P, et al. Barley mutants lacking chloroplast glutamine synthetase-biochemical and genetic analysis. Plant Physiol, 1987, 83: 155–158
Edwards J W, Walker E L, Coruzzi G M. Cell specific expression in transgenic plants reveals nonoverlap** roles for chloroplast and cytosolic glutamine synthetase. Proc Natl Acad Sci USA, 1990, 87: 3459–3463
Zhao X Q, Shi W M. Expression analysis of the glutamine synthetase and glutamate synthase gene families in young rice (Oryza sativa) seedlings. Plant Sci, 2006, 170: 748–754
Blackwell R D, Murray A J S, Lea P J, et al. Photorespiratory amino donors, sucrose synthesis and the induction of CO2 fixation in barley deficient in glutamine synthetase and/or glutamate synthase. J Exp Bot, 1988, 39: 845–858
Somerville S R, Ogren W L. Inhibition of photosynthesis in Arabidopsis mutants lacking leaf glutamate synthase activity. Nature, 1980, 286: 257–259
Coschigano K T, Melo-Oliveira R, Lim J, et al. Arabidopsis gls mutants and distinct Fd-GOGAT genes: Implications for photorespiration and primary nitrogen metabolism. Plant Cell, 1998, 10: 741–752
Ferrario-Mery S, Suzuki A, Kunz C, et al. Modulation of amino acid metabolism in transformed tobacco plants deficient in Fd-GOGAT. Plant Soil, 2000, 221: 67–79
Hausler R E, Blackwell R D, Lea P J, et al. Control of photosynthesis in barley leaves with reduced activities of glutamine synthetase and glutamate synthase: Plant characteristics and changes in nitrate, ammonium and amino acids. Planta, 1994, 194: 406–417
Blackwell R D, Murray A J S, Lea P J. Inhibition of photosynthesis in barley with decreased levels of chloroplastic glutamine synthetase activity. J Exp Bot, 1987, 38: 1799–1809
Carvalho H G, Lopes-Cardoso I A, Lima L M, et al. Nodule-specific modulation of glutamine synthetase in transgenic Medicago truncatula leads to inverse alterations in asparagines synthetase expression. Plant Physiol, 2003, 133: 243–252
Harrison J, Pou M A, Sene O, et al. Does lowering glutamine synthetase activity in nodules modifying nitrogen metabolism and growth of Lotus japonicus? Plant Physiol, 2003, 133: 253–262
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported in part by grants from the National Basic Research Program of China (Grant No. 2005CB120905), the National Special Key Project of China on Functional Genomics of Major Plants and Animals, the National Natural Science Foundation of China and the Cultivation Fund of the Key Scientific and Technical Innovation Project, Ministry of Education of China (Grant No. 707045).
Electronic supplementary material
About this article
Cite this article
Cai, H., **ao, J., Zhang, Q. et al. Co-suppressed glutamine synthetase2 gene modifies nitrogen metabolism and plant growth in rice. Chin. Sci. Bull. 55, 823–833 (2010). https://doi.org/10.1007/s11434-010-0075-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11434-010-0075-9