Abstract
Purpose
The main objective of the study was the determination of the Cd, Cr, Cu, Pb, and Zn distribution in Wigry Lake sediments, as well as the contamination and ecotoxicological risk assessment on the basis of a large data set.
Materials and methods
Select metal concentrations were determined via AAS or ICP-MS. Contamination assessment was achieved via the implementation of different geochemical background values, selected pollution indices, and by way of comparison with the limit values of the sediment quality guidelines and supported by cartographic methods.
Results and discussion
Metal concentrations presented in the paper were associated with a specific type of sediment and sedimentation environment. The highest concentrations of metals were found in the fluvial-lacustrine sediment covering the bottom of the bay of eutrophic character. The lowest amounts were found in the lacustrine chalk and clastic sediment present in the littoral parts of the lake, while profundal sediments were more enriched with metals. Studies revealed that the examined metals have mostly natural, but also anthropogenic origin. The assessment of lake sediments, with the use of geochemical background values and different indices, yielded highly diversified results associated with the variability of background values applied in the study. However, ultimately, the Wigry Lake sediments were found to be only slightly contaminated with Cd, Cr, Cu, and Zn, while Pb concentrations were considered to be more disturbing. The potential ecotoxicological risk was assessed as low.
Conclusions
Particular attention in this study was paid to the significance of the geochemical background values adopted for calculations, which, in the case of Wigry Lake, gave very divergent results. A uniquely large data set facilitated the performance of a thorough analysis of metal distributions in recent lacustrine sediments and highlighted the necessity of using integrated approaches in aquatic ecosystem studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Aquatic sediments are a very important part of water ecosystems. They play a significant role in the circulation of elements between various components of water and ground systems. They can also act as a natural filter and indicator of the environmental degradation degree (Burton et al. 2005; Singh et al. 2005; Superville et al. 2014; Tylmann et al. 2017). Sediments deposited recently are frequently characterized by a higher content of some contaminants in comparison with sediments accumulated several hundred years ago (Salomons and Förstner 1984). Among the inorganic compounds, special attention is paid to metals which are characterized by potential toxicity, nonbiodegradability, persistent nature, and bioenrichment ability in the food chain, as well as the facility with which they can migrate over long distances (Szyczewski et al. 2009; Nobi et al. 2010). Metals which are present in higher concentrations disrupt the biological balance in water reservoirs (Wardas et al. 2010; Wilk-Woźniak et al. 2011; Yi et al. 2011; Milošković et al. 2013) and potentially represent a serious threat to ecosystems and human health (Zhang et al. 2017; Dai et al. 2018; Islam et al. 2018).
There has been considerable progress worldwide in the field of sediment quality assessment since 2000. Technical guidelines on methods for determining the quality of aqueous sediments were provided by the European Union as a part of the Water Framework Directive (EU WFD 2011). Generally, two methods are used to assess the status of sediment contamination: (1) comparison of element concentrations in a sediment with geochemical background concentrations, and (2) comparison with sediment quality guidelines and cumulative effects of contaminants in sediments (Reimann et al. 2005; Gałuszka and Migaszewski 2011; Simpson et al. 2013).
Numerous geochemical studies have contributed to the establishment of metal background values that can be used for environmental quality evaluation (Matschullat et al. 2000). The main approaches to geochemical background concentration determination include empirical (geochemical), theoretical (statistical), and integrated (combined geochemical and statistical) methods (Dung et al. 2013). Many researchers use the large-scale geochemical background proposed by Taylor and McLennan (1995) as the average concentration of elements in the upper continental crust, by Turekian and Wedephol (1961) as the average concentration in the shale, or by Håkanson (1980) as the global preindustrial sediments. In such situations, local conditions are often not considered, which causes large deviations in assessment results (Xu et al. 2017). This approach has even more limitations pointed out by Reimann and de Caritat (2000, 2005), where the most important of them are (1) natural diversity in the chemical and mineralogical composition of studied rocks or other environmental component and reference crust rocks used for calculations, (2) underestimation of the importance and influence of naturally occurring processes like weathering, erosion, transport, or biogeochemical processes. Therefore, the clarification of regional conditions or levels of basic metals is necessary for environmental assessment and legislation (Matschullat et al. 2000). One of the main methods for the determination of the regional/local background values is based on using the raw element data from surface sediment samples, rejecting the values corresponding to contamination and calculating the statistical mean and standard deviation (Chen et al. 2001; Xu et al. 2017). Another method is to use the concentration of elements in deep, preferably dated, core sediments (Cobelo-García and Prego 2003; Gałuszka and Migaszewski 2011). However, this approach is not flawless either, as element concentrations in different components of a particular environment may be naturally subject to change due to biogeochemical processes (Matschullat et al. 2000; Reimann and de Caritat 2005).
The distribution of metals in aquatic sediments is controlled by a variety of factors, including sediment texture, source-rock mineralogy of rocks in the catchment, and geochemical processes present during transport, deposition, and postdeposition (Bábek et al. 2015; Matys Grygar and Popelka 2016; Thin et al. 2020). Their accumulation in bottom sediments, as a result of complex physical and chemical adsorption onto inorganic and organic particulates, depends on the sediment matrix and properties of bounded elements. In lacustrine sediments, the level of the natural concentration of metals depends mostly on the geological structure of the catchment from which a material is transported and on the properties of sediments, mainly on the composition of grain-size fractions and organic matter content (Boyle 2001; Tylmann et al. 2011; Lin et al. 2016).
The most commonly used geochemical methods for metal contamination quantification include the geoaccumulation index (Igeo) by Müller (1969), enrichment factor (EF), and contamination factor (Cf) (Håkanson 1980; Sutherland 2000; Boës et al. 2011), which refer to the geochemical background values. The assessment of the potential eco-risk is performed using sediment quality guidelines (SQGs) and potential ecological risk indices (Er and RI) (Håkanson 1980; MacDonald et al. 2000). Such factors as the establishment of aquatic sediment quality guidelines and development of different geochemical and ecotoxicological criteria have resulted in the evaluation of bottom sediment quality in many countries (e.g., Varol 2011; Selvam et al. 2012; Wang et al. 2012; Zhang et al. 2014; Soliman et al. 2015; Ouchir et al. 2016; Aleksander-Kwaterczak and Plenzler 2019; El-Kady et al. 2019). However, the SQGs do not reflect in situ conditions, as sediment chemistry is diversified and pollutants bioavailability depends on physiochemical properties of deposits and local conditions, which additionally may be easily altered during the sampling and operation processes. Every method for metal contamination assessment has its advantages and disadvantages. Therefore, aquatic ecosystems which include sediments should be assessed using integrated techniques (MacDonald et al. 2000; Yao and Gao 2007; Dung et al. 2013).
The main objective of the study was to determine the concentration of selected metals (Cd, Cr, Cu, Pb, and Zn) in Wigry Lake sediments and to assess the degree of contamination of these deposits, on the basis of large data sets. As the lake has been an object of scientific interest for many years, abundant literature describing its environment can be found. The lake sediments were thoroughly recognized thanks to physiochemical and cartographic and seismic surveys (Król 1998; Rutkowski et al. 2002a, 2002b, 2003, 2005, 2007, 2008, 2009; Rutkowski 2004). The Holocene history of the Wigry Lake and its surroundings was reconstructed via the research of sediment cores and radiometric dating (Pawlyta et al. 2004; Kupryjanowicz 2007; Piotrowska et al. 2007; Rutkowski et al. 2007; Zawisza and Szeroczyńska 2007; Aleksander-Kwaterczak et al. 2009). Metals were widely studied for both total content and chemical speciation (Prosowicz and Helios-Rybicka 2002; Aleksander-Kwaterczak and Prosowicz 2007; Helios-Rybicka and Kostka 2007; Aleksander-Kwaterczak et al. 2009; Aleksander-Kwaterczak and Kostka 2011). The distribution of individual elements was also demonstrated using the methods of geographic information system and geostatistics (Kostka et al. 2008; Kostka 2009; Kostka and Leśniak 2020). Kostka and Leśniak (2020) gathered and summarized long-time research concerning three metals (Fe, Mn, and Zn), focusing on the graphic imaging of the distribution of these metals and their association with sediment types. In the current article, the contribution of anthropogenic activities to the level of metal contamination was assessed using geochemical background values and different contamination indices. The obtained results were also compared with limit values presented in the SQGs. Particular attention was paid to the significance of the approach to geochemical background determination and its values adopted for calculations.
2 Study area characterization
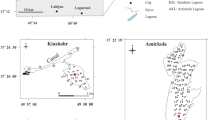
The Wigry Lake (NE Poland) is one of the deepest (up to 73 m) and largest (21.2 km2) of the Polish lakes. It has a diverse shape and shoreline, with a relatively high (4.35) degree of coastline development. Its geomorphology was formed by the Vistulian glaciation and is highly complicated. The lake consists of five visibly different parts (Fig. 1): the Zakątowskie and Bryzglowskie Basins (of a morainic type), Szyja Basin and Wigierki Bay (of a furrow type), and Wigierskie Basin (partly morainic and furrow). Its bathymetry is also quite complicated (Fig. 2). The waters of the lake are supplied mainly by the Czarna Hańcza and Wiatrołuża rivers, which flow into Wigry Lake from the north.
Bottom sediments of the lake have been analyzed for over a century, but detailed research began only in 1997, lasted for over a decade and resulted in the obtaining of about 1200 sediment cores of different lengths. This has facilitated accurate physiochemical (Table 1), as well as cartographic and seismic recognition of those deposits (Król 1998; Rutkowski et al. 2002a, 2002b, 2003, 2005, 2007, 2008, 2009; Rutkowski 2004). In the course of subsequent research of the Wigry Lake, samples were analyzed for different scientific purposes, and not all sediments properties were determined for all sampling points, hence, the differences in the number of results for individual elements presented in this study.
Sedimentation in the Wigry Lake (Table 1, Fig. 2) is dominated by carbonate deposits which reflect the petrographic composition of rocks building postglacial surroundings of the lake. It is mainly represented by lacustrine chalk and carbonate gyttja. The lacustrine chalk covers about 25–30% of the littoral zone bed and basically corresponds to the extent of offshore and inlake shallows, the range of which was determined through meticulous analysis of high-resolution aerial photographs (Rutkowski 2004). This type of sediment is richest in calcium carbonate, with a slight admixture of organic matter, low water content, and high volumetric density (Table 1). The profundal zone, which covers about 60–75% of the lake’s bottom, is primarily represented by fine-grained carbonate gyttja, richer in organic matter, and poorer in calcium carbonate when compared with lacustrine chalk. Organic gyttja is present in some isolated bays and eutrophication-exposed zones of the lake (Cieszkinajki and Krzyżańska Bays; about 2–3% of the lake surface). Organic matter is a significant component of this sediment, while the calcium carbonate concentration is lower. This sediment is heavily watered and its bulk density is very low. The specific fluvial-lacustrine sediment is located at the mouth of the Czarna Hańcza River. It is distinguished by a characteristic appearance of poorly decomposed plant remains, very dark (almost black) color, and intensive smell, but its physiochemical characteristic is quite similar to organic gyttja. Therefore these two types of sediment have been combined in Table 1. Clastic sediments appear locally in coastal zones in the form of narrow belts and constitute approximately a few percent of the lake’s surface. These are mainly sands and gravels (Prosowicz and Helios-Rybicka 2002; Rutkowski et al. 2002a, 2003, 2007, 2008; Rutkowski 2004; Aleksander-Kwaterczak and Prosowicz 2007; Aleksander-Kwaterczak et al. 2009).
The fact whether a particular deposit sample belongs to one of five distinguished categories was organoleptically assessed during sampling (as sediments differed, e.g., in color and consistency) and then verified on the basis of laboratory studies. However, there are no clear boundaries between particular sediment types, and their properties change smoothly in space, while some samples have intermediate properties. Moreover, untypical sediments may also occur in areas of the occurrence of some other deposits. For example, carbonate gyttja can sometimes be found in the littoral zone, especially in the eutrophicated areas (as a symptom of increasing trophy of the lake), while lacustrine chalk can be observed at depths greater than 10 m. Deposits located near shores and offshore shallows may also be disturbed by fishermen, and landslides disrupting natural sedimentation have been observed in some areas featuring steep slopes.
The lake is surrounded by small villages with agricultural areas and forests (Fig. 1). The catchment area of the Wigry Lake is 453.7 km2, while 148.4 km2 is protected within the borders of the Wigry National Park (WNP) created in 1989. About 67% of WNP is covered by forest, 19% by water, and 14% by agricultural land. The Czarna Hańcza River flows through the city of Suwałki (located approx. 5 km to the northwest, having about 70,000 inhabitants), where sewage from municipal sewage treatment plants is discharged into the river. Moreover, areas beyond city limits are not covered by any organized sewage collection system (Kostka 2009; Kostka and Leśniak 2020).
3 Materials and methods
3.1 Sample collection
Sediment samples were collected during the summer stagnation periods over the course of more than a decade and covered the entire area of Lake Wigry in an irregular grid, obtaining a sampling density from approx. 8 (for lead) to approx. 21 (for zinc) samples per km2. Sediments were collected from the bottom of the lake using a sampler equipped with a transparent plastic tube, in which an undisturbed sediment core (several cm of diameter; up to 1.5 m long) is pressed by gravity (Rutkowski 2007). Their precise location was determined using Global Positioning System (GPS) navigation. The depths from which the samples were taken were measured by an FCV echo-sounder (model 381/382 by Furuno), sometimes also using a YSI model 6920 multiparameter water quality monitor, and, in the shallows, with a calibrated pole. Sediment cores were transported to the laboratory inside plastic tubes, then carefully removed and sliced into subsamples. The upper 0–5 cm of sediment cores (considered to represent the recent deposits) were taken for further geochemical analysis.
3.2 Analytical methods
The concentration values of selected elements, i.e., Cd, Cu, Cr, Pb, and Zn in upper 0–5 cm of sediment cores, were analyzed using atomic absorption spectrometry (AAS – PYE UNICAM SP9; Pb–LOQ = 10 μg l−1 and Zn–LOQ = 1 μg l−1), or using an inductively induced mass spectrometer (ICP-MS – HP 4500; Cd–LOQ = 0.16 μg l−1, Cr–LOQ = 0.21 μg l−1, and Cu–LOQ = 0.23 μg l−1), following previous extraction in a microwave oven (MDS 2000), using 10 ml of 65% HNO3 and 2 ml of 30% H2O2 (with the highest degree of purity), successively. In order to ensure analytical accuracy, reagent blanks were used, LSKD-4 reference material was analyzed for every tenth sample, and selected elements were additionally determined using inductively coupled plasma atomic emission spectrometry (ICP-AES – Perkin-Elmer Plasma 40) (Table 2). The evaluation of the results quality (determined as very good) was carried out using the analysis of variance by the Robust statistics method (Ramsey et al. 1992; Ramsey 1993). Descriptive statistics (Table 3) were calculated using the Statistica software (13.3) and cumulated distribution functions of particular metal concentrations in Wigry Lake sediments (Figs. 3, 4, 5, 6, and 7) were prepared with the use of the R language.
Cumulated distribution functions of cadmium concentration in a clastic sediment, b lacustrine chalk, c carbonate gyttja, d organic gyttja, e fluvial-lacustrine sediment, f all sediments; circular symbols depict five different geochemical background values used in this study (Table 4), while rhombus symbols depict SQGs values (Table 5) (data in brackets are not shown as was not determined (GB2) or their values are beyond the graphs scale (PEL, PEC))
Cumulated distribution functions of chromium concentration in a clastic sediment, b lacustrine chalk, c carbonate gyttja, d organic gyttja, e fluvial-lacustrine sediment, f all sediments; circular symbols depict five different geochemical background values used in this study (Table 4), while rhombus symbols depict SQGs values (Table 5) (data in brackets are not shown as was not determined (GB2) or their values are beyond the graphs scale (TEL, TEC, PEL, PEC))
Cumulated distribution functions of copper concentration in a clastic sediment, b lacustrine chalk, c carbonate gyttja, d organic gyttja, e fluvial-lacustrine sediment, f all sediments; circular symbols depict five different geochemical background values used in this study (Table 4), while rhombus symbols depict SQGs values (Table 5) (data in brackets are not shown as their values are beyond the graphs scale)
Cumulated distribution functions of lead concentration in a clastic sediment, b lacustrine chalk, c carbonate gyttja, d organic gyttja, e fluvial-lacustrine sediment, f all sediments; circular symbols depict five different geochemical background values used in this study (Table 4), while rhombus symbols depict SQGs values (Table 5) (data in brackets are not shown as their values are beyond the graphs scale)
Cumulated distribution functions of zinc concentration in a clastic sediment, b lacustrine chalk, c carbonate gyttja, d organic gyttja, e fluvial-lacustrine sediment, f all sediments; circular symbols depict five different geochemical background values used in this study (Table 4), while rhombus symbols depict SQGs values (Table 5)
3.3 Geochemical backgrounds and contamination assessment
Five different geochemical backgrounds were applied in the present study (Table 4). Three of them were local and the first one (GB1) was defined as mean concentration based on five samples of lacustrine chalk underlying peat dated with the 14C method at 7970 ± 70 years BP (Król 1998; Prosowicz and Helios-Rybicka 2002). The second one (GB2) was adopted from Migaszewski et al. (2003) as a range of metal concentrations in bottom layer of Wigry Lake sediment cores (up to 120 cm long). Although several other studies related to the sediment cores of the lake, including metals (e.g., Aleksander-Kwaterczak and Prosowicz 2007; Rutkowski et al. 2007; Aleksander-Kwaterczak et al. 2009; Aleksander-Kwaterczak and Kostka 2011), data presented by Migaszewski et al. (2003) were considered the most suitable for the determination of natural concentration levels. Cores were taken from all five main parts of the lake (Fig. 1), and their bottom, deeply buried layers were approximately dated from 485 to 2172 years, which means that they originate from the preindustrial period. The third local geochemical background (GB3) was based on the measured values of individual metal concentrations in the recent Wigry Lake sediments. It was determined with the use of a method called the “calculated distribution function” proposed by Matschullat et al. (2000). This method assumes that, for a given environment, the natural distribution of metal concentration values is the normal one and the abnormal values (usually associated with contamination) lead to right-skewed distribution. This results in the presence of more samples with above-average values. It was found that all samples of the Wigry Lake sediments, when treated as one data set, did not meet the assumption of this method; therefore, calculations were done separately for different sediment types. Nevertheless, the number of samples of organic gyttja, fluvial-lacustrine, and clastic sediments were too small, and, in the case of lacustrine chalk, the method assumption was met only for Pb and Cr. The only sediment type characterized by the normal distribution with right-tailed skewness for all metals was carbonate gyttja. Therefore, final calculations based on the method quoted above were carried out for this sediment type data. The method assumes that values greater than the threshold equal to the median increased by 2σ are anomalous, while values smaller than this threshold represent background values. The σ value is the standard deviation of a normal distribution estimated on the basis of data lower than the median value (Matschullat et al. 2000).
The geochemical background for Polish aquatic sediments (GB4), adopted from Lis and Pasieczna (1995), was statistically determined on a basis of an abundant data set (993) constituting aquatic sediment samples taken from various environments, such as rivers, streams, channels, lakes, reservoirs, or ponds. Finally, the world geochemical background for carbonates (GB5) was used, as proposed by Turekian and Wedephol (1961).
The contamination factor (Cf; by Håkanson 1980) was used to describe sediment contamination by an individual toxic substance. It was calculated according to Eq. 1. The classification was established as follows: low contamination (Cf < 1), moderate contamination (1 ≤ Cf < 3), considerable contamination (3 ≤ Cf < 6), and very high contamination (Cf ≥ 6).
where Ci is the mean concentration of the substance i from at least five sample sites, and Bin is the preindustrial reference level for this substance.
The degree of contamination (Cd; by Håkanson 1980) is defined as the sum of all contamination factors (Cif). It provides information on the total contamination (Eq. 2). Cd < 8 indicates a low degree of contamination, 8 ≤ Cd < 16 a moderate degree of contamination, 16 ≤ Cd < 32 a considerable degree of contamination, and Cd ≥ 32 a very high degree of contamination.
The pollution load index (PLI; by Tomlinson et al. 1980) was used to detect contamination by metals in each of the respective sample locations. It was calculated according to equation 3. The value of PLI < 1 indicates no pollution, then 1 ≤ PLI < 2 moderate pollution, 2 ≤ PLI < 3 heavy pollution, and PLI ≥ 3 extremely heavy pollution (Tomlinson et al. 1980; Rashki Ghaleno et al. 2015).
where CF is the concentration factor for an individual metal: CFn = C/Bn; C is the metal concentration in the analyzed sample site, and Bn is the preindustrial reference level for the substance.
The SQGs were used to assess sediment quality conditions and the impact of contaminated sediments on aquatic organisms based on a threshold effect concentration (TEC) and probable effect concentration (PEC) (MacDonald et al. 2000). Additionally, we implemented the probable effect level (PEL) and the threshold effect level (TEL) values (Smith et al. 1996), which delineated three ranges of chemical concentrations: rarely (≤ TEL; minimal effect range), occasionally (> TEL and ≤ PEL; possible effect range), and frequently associated with adverse biological effects (> PEL; probable effect range) (Table 5).
3.4 Cartographic analysis
All maps presented in this study were prepared with the use of the ESRI’s ArcGIS Pro software. The bathymetry map (Fig. 2) was created on the basis of depth values measured at individual sampling points. The choice of an appropriate interpolation method depends on several factors, most important of which are: the existence of an analyzed phenomenon physical model, nonzero autocorrelation of the spatial variable, and its spatial correlation with other factors or the expected results of the prediction procedure (Pebesma et al. 2007). The preliminary analysis of the analyzed data demonstrated significant variation between the values. Moreover, it was observed that the depth values were variables with nonzero autocorrelation. In this case, the best unbiased estimator of unknown function values at the point where the measurement was not performed is ordinary kriging (Burrough and McDonnell 1998). The interpolation procedure for the depth value dataset was carried out in several successive steps. First, data stationarity was checked, i.e., a spatial trend indicating their nonstationary character was not found. The data distribution compliance with normal distribution was subsequently verified using quantile–quantile (Q-Q) plots (Gan and Koehler 1990). Since the depth values differed significantly from the normal distribution, their transformation to this distribution was carried out using a Box-Cox transformation with λ = 1.0, which gave acceptable results. The next stage of the analysis was the estimation of anisotropic, empirical semivariograms, which quantifies the spatial relationships between the data. The empirical semivariogram was then approximated by a theoretical semivariogram, using nonlinear regression methods, so as to achieve the best fit (minimum root mean square error). The theoretical semivariogram was used to estimate depth values in regular grid points, according to ordinary kriging procedure.
The bathymetry map was used as the background to present an approximated extent of five general bottom sediment types identified in Wigry Lake. The Thiessen polygon function was used to generate the map. The procedure assumes that, in the area bounded by a particular polygon, there is one sediment type identified in the single measuring point lying inside it. In this way, five deposit types (clastic sediment, lacustrine chalk, carbonate gyttja, organic gyttja, and river-lake sediment) were presented in the form of a map (Fig. 2). Data presentation on the map depicting Wigry Lake sediments according to local geochemical background (GB3) (Fig. 8) was determined by the recommendations of McKinley et al. (2016). Maps showing the sediment classification in terms of the PLI parameter (Fig. 9) were obtained through the interpolation of PLI values calculated for each sampling point by Eq. 3 (in the case of GB2, the upper range was taken for calculations).
Wigry Lake bottom sediments mapped according to PLI index and regarding five different geochemical backgrounds (Table 4): local a (GB1); local b (GB2); local c (GB3); Polish, for aquatic sediments (GB4); world, for carbonates (GB5)
4 Results and discussion
Concentration values for all examined metals were the highest in the case of organic-rich sediments – especially the fluvial-lacustrine sediment and organic gyttja. The lowest concentration values were generally observed in the case of clastic sediment or lacustrine chalk (Table 3, Figs. 3, 4, 5, 6, and 7), which may be the result of very low mineralization and chemical composition of the overlying water. According to Aleksander-Kwaterczak and Zdechlik (2016), mineralization of lacustrine chalk pore solutions was significantly lower in comparison with other types of sediments. The other reason for this phenomenon is the chemical and granular composition of deposits. The comparison of arithmetic means and median values in various sedimentation environments revealed that those values were usually higher in the profundal zone, where clayey sediments predominate, and it is known that the accumulation of metals in fine fractions is generally higher than in coarse sediments. This phenomenon was confirmed by numerous authors (e.g., Salomons and Förstner 1984; Selvaraj et al. 2004; Tylmann et al. 2011; Kuriata-Potasznik et al. 2016; Matys Grygar and Popelka 2016) and was discussed more extensively by Kostka and Leśniak (2020), with reference to the Wigry Lake. Sedimentation conditions also explain the positive correlation between metal concentration levels and lake depth (Kostka 2009; Kostka and Leśniak 2020) observed for four of five metals discussed: Cd-depth−0.34, Cr-depth−0.46, Cu-depth−0.49, and Zn-depth−0.58. The fluvial-lacustrine sediment (accumulated in the mouth of Czarna Hańcza River, Wigierskie Basin) and, to a lesser extent, organic gyttja (accumulated in Cieszkinajki Bay and Krzyżańska Bay) represent some specific exceptions to that phenomenon, as those sediments were significantly contaminated despite being deposited at minimal depths (Fig. 2, Table 3). In the case of these deposits, the main factor favoring metal accumulation is most probably the relatively high organic matter content (Table 1). This phenomenon was already characterized by many authors (e.g., Coquery and Welbourn 1995; El Bilali et al. 2002; Migaszewski et al. 2003; Salam et al. 2019) and previously described more closely in the context of Wigry Lake sediments (Kostka and Leśniak 2020). Moreover, Cieszkinajki Bay and Krzyżańska Bay are relatively isolated from the main lake basin (Fig. 1), which restricts water circulation and exposes those areas to eutrophication. In the case of the fluvial-lacustrine sediment, the river-lake system probably acts as a filter and contributes to flowing river purification (Helios-Rybicka and Kostka 2007; Kuriata-Potasznik et al. 2016), which was confirmed by previous studies of the Czarna Hańcza River-Wigry Lake system (Migaszewski et al. 2003; Zdanowski 2003).
All the above considerations apply basically to all examined metals, but mostly to Cd, Cr, Cu, and Zn. Concentration values for these metals seem to be affected by similar phenomena, which is confirmed by appropriate correlation coefficients (Cd-Cr−0.71, Cd-Cu−0.77, Cd-Zn−0.55, Cr-Cu−0.85, Cr-Zn−0.65, Cu-Zn−0.60), while Pb has slightly different geochemical characteristics. The highest mean concentration values of lead were found in the fluvial-lacustrine sediment, and in the case of lacustrine chalk (Table 3, Fig. 6). Mean Pb concentration values in different sediment types were also the least diversified. This metal was weakly and negatively correlated with depth (r = −0.21) and did not correlate with Cd and Zn. Correlation coefficients for Cr and Cu could not be calculated, as Pb concentration values were determined on a separate data set. Lead is known to be poorly mobile, and when released during weathering processes, it is easily accumulated and immobilized in carbonates (Hamilton-Taylor and Davison 1995; Migaszewski et al. 2003; Aleksander-Kwaterczak and Kostka 2011; Bojakowska et al. 2014), especially at pH > 6.5 and the pH value of Wigry Lake sediments, particularly within the littoral zone, is clearly alkaline (Migaszewski et al. 2003).
The main purpose of this article was to assess the contamination of Wigry Lake bottom sediments with metals, taking into account the critical review of geochemical background values and different pollution indices. Background values are considered to be essential in determining the levels of contamination that affect freshwater ecosystems (Gałuszka 2007; Turner et al. 2017), and since they usually constitute a component of different pollution indices used in environmental studies, their proper determination is crucial. In the case of Wigry Lake, five different geochemical backgrounds were applied (Table 4). The local backgrounds−GB1 (Król 1998; Prosowicz and Helios-Rybicka 2002) and GB2 (Migaszewski et al. 2003)−were determined for lacustrine sediments deeply buried and isolated from anthropogenic influences. In both cases, the “reference” sediment had a similar origin and physiochemical properties as lacustrine chalk covering the surface littoral part of the current lake. Although GB1/GB2 seems to be the most appropriate, it should be remembered that lacustrine chalk, which has the main share in fossil sediments, currently covers only about 25% of the lake surface (Fig. 2), thus is not fairly representative of the whole current lake environment area. It should also be noted that GB1 and GB2 values vary a lot (Table 4), even though they were established “very locally” and in a similar way. Both GB1 and GB2 values were determined on the basis of five samples. However, in the case of GB2, sampling points were more spatially spread, and the background values were given in the form of a range, which is considered to be more appropriate than just a single value (Matschullat et al. 2000). Therefore, GB2 seems to be more accurate of those two values. On the other hand, differences in analytical methods used for determining metal concentration values applied by Migaszewski et al. (2003) and during the present study, including different extraction reagents and separation of a particular grain fraction, are the weakness of GB2. The abovementioned discrepancies also concern the background value for Polish aquatic sediments (GB4) determined by Lis and Pasieczna (1995) and the world geochemical background (GB5) for carbonates (Turekian and Wedephol 1961). Moreover, GB4 was established on a basis of a set of samples of different origins (i.e., rivers, streams, lakes, etc.) without taking into account the sediment type. Reimann and de Caritat (2000) pointed out the varied solubility of sediments building minerals in chemicals used in environmental studies as one of the pollution assessment weaknesses. Finally, the world geochemical background (GB5), as generally defined for carbonates, seems to be insufficiently precise. Any geochemical background other than a local one is considered to be meaningless, as background concentrations are regionally variable and are a function of time (Matschullat et al. 2000). Furthermore, regional or world geochemical backgrounds do not consider local sedimentation conditions and the nature of the catchment. The local geochemical background (GB3) calculated as proposed by Matschullat et al. (2000) seems to be devoid of most of the disadvantages discussed above. It was calculated on the basis of data presented in the current study; therefore, analytical methodology was consistent, and it was established as locally as possible. It should be mentioned that, although we had enough data to carry out this kind of analysis, we encountered some difficulties, mainly due to the diversification of sediment types occurring in the lake, which contributes to the differentiation of metal concentration levels (Table 3, Figs. 3, 4, 5, 6, and 7). In that case, data could not be treated as one data set and calculations needed to be done for different sediment types separately. In the case of organic gyttja, fluvial-lacustrine, and clastic sediment, geochemical background values could not be calculated using this method, as the number of samples was too small. In the case of lacustrine chalk and carbonate gyttja, data sets were abundant enough, but only in the case of carbonate gyttja, the method’s assumptions were met for all examined metals. Finally, we decided to calculate GB3 values (Table 4) only for carbonate gyttja. However, since it covers the majority of the Wigry Lake bottom, it may be assumed as representative for the current lake environment. All relevant background values have been depicted in graphs (Figs. 3, 4, 5, 6, and 7) and Wigry Lake samples have also been mapped in Fig. 8 according to GB3, as was considered to be the most appropriate in the course of the present study.
Being aware of the limited usefulness of some geochemical background values, we still decided to apply them in further calculations, mainly for comparative purposes. As mentioned above, different pollution indices are usually based on GB values, thus their proper definition and determination is crucial (Reimann and de Caritat 2005; Xu et al. 2017). In Table 6, we have presented the results for Cf (contamination factor; referring to a single metal) and Cd (degree of contamination, referring to summarized metal contamination), proposed by Håkanson (1980). As Cd is simply just a sum of Cfs, its value is strictly influenced by the number of metals being examined. We then used PLI (pollution load index), proposed by Tomlinson et al. (1980), in order to assess combined metals contamination (Fig. 9).
It is not surprising that very large discrepancies were found in terms of the contamination assessment of Wigry Lake sediments, in the context of both the geochemical background values and pollution indices. The lowest geochemical background values were established for GB1, while the highest ones generally for GB3, except for Cr, in which case the GB5 value was the highest. Taking into account all lithological types of sediments, the biggest differences in the obtained Cf values (Table 6) were found for lead (0.9–328.4) and cadmium (0.5–124.0). The highest obtained Cf values are derivatives of GB1, and, as they are much higher than 6 (the limit value indicating very high contamination), they seem to be nearly ridiculous. The same applies to the Cd value calculated according to GB1 (478.9) and being well above 32, which indicates a very high degree of contamination. The obtained results were also consistent with the PLI representation (Fig. 9). When considering particular sediment types, the observed results were even higher, especially in the case of the fluvial-lacustrine sediment. As mentioned above, GB1 is not representative of the current Wigry Lake environment, but even if we consider only lacustrine chalk, the percentages of samples with particular metal concentration levels exceeding GB1 values were still high, i.e., 60% for Cr, 83% for Cu, and 100% for Cd, Pb, and Zn, and relevant Cf and Cd values were still extremely high (Table 6). The values of GB2, GB4, and GB5 are quite similar for Cu, Pb, and Zn providing similar contamination index values, while other geochemical backgrounds and related indices differ to a various extent (Table 6, Fig. 9). The most interesting is GB3 calculated according to Matschullat et al. (2000), which seems to be the most appropriate, as it is considered to be devoid of most of the weaknesses related to other backgrounds. Its values are basically the highest (except for Cr) and the closest to GB4 values (Table 4). The Cf indices calculated according to GB3 were generally below 1, indicating low contamination (Håkanson 1980) for all sediments, as well as their particular types. The exception was the fluvial-lacustrine sediment for which the Cf and Cd indices implicated moderate to considerable contamination (Table 6). These results are also reflected in Fig. 9, where the vast majority of the Wigry Lake area is covered by sediments characterized by the PLI index lower than 1 (when calculated according to GB3), which indicates the absence of pollution (Tomlinson et al. 1980). A small area with higher PLI values corresponds to the extent of the fluvial-lacustrine sediment. The authors are aware of the fact that GB3 was calculated only on a basis of calcium carbonate gyttja samples; however, this type of deposit prevails in the current Wigry Lake sediment; thus, it can be considered sufficiently representative, although not necessarily for the highly organic fluvial-lacustrine sediment.
Concentration values of the examined metals were also compared with the limit values recommended by the sediment quality guidelines (Smith et al. 1996; MacDonald et al. 2000) and were found not to pose a serious threat to living organisms. In the case of Cr, none of the threshold values were exceeded, even for a single sample (Tables 3 and 5, Fig. 4). The TEL and the TEC values, but not the PEL and PEC values, were exceeded for Cd and Cu concentration values, and relevant percentages of samples were low: 14% and 3% (for Cd), 2% and 2% (for Cu), respectively (Tables 3 and 5, Figs. 3 and 5). About 96% of samples had a Pb concentration value higher than TEL and TEC values but just only 2% higher than PEL value (Tables 3 and 5, Fig. 6). In the case of Zn, samples exceeding all SQGs values were found, however relevant percentages were low: 4% (TEL and TEC), 3% (PEL), and 1% (PEC) (Tables 3 and 5, Fig. 7).
A comparison of the concentration of examined metals in sediments with other lakes, both in Poland and in other parts of the world (Table 7), may be a good indicator of the Wigry Lake chemoecological state. When searching the literature for comparison, we tried to choose lakes located in different parts of the world, but of a similar genesis. Attention was also paid to the abundance of data sets, and whether samples were collected from different depths. However, the comparison may only be an estimate, due to the lack of a standardized methodology for sediment research used in various countries and institutions and due to the natural variability of lake environments worldwide. Considering Cd and Zn, most of the compared lakes are characterized by lower levels of concentration of these elements than those found in Wigry Lake. However, it is worth noting that this result is mainly affected by very high values of metal concentration found in sediment deposited near the mouth of the Czarna Hańcza River to the lake, which covers a very small area of the bottom, while most of the analyzed sediments are much less enriched with examined metals. Taking into consideration Cu and Pb, Table 7 includes lakes both more and less contaminated with these metals, while in case of Cr only Gopło Lake (Poland) is less enriched with this element.
Taking into account all the above considerations, we can assume that the Wigry Lake environment is only slightly contaminated with the analyzed metals. Although recent lake sediments are enriched with all examined elements, as reported by sediment core studies (Migaszewski et al. 2003; Aleksander-Kwaterczak and Prosowicz 2007; Rutkowski et al. 2007; Aleksander-Kwaterczak et al. 2009; Aleksander-Kwaterczak and Kostka 2011) and the trophy of the lake has been rising over the last century, with the highest rate in the 1960s–1990s (Rutkowski et al. 2007), the majority of presented data does not indicate a serious threat to the chemoecological condition of the lake. The province in which Wigry Lake is located is the least populated in Poland (GUS 2019), the lake catchment is not affected by either metal mining or any heavy industry and the Wigry National Park together with Białowieża National Park and Biebrza National Park belongs to the most pristine regions of Europe, called the green “lungs” of Poland (Migaszewski et al. 2003). Therefore, the Wigry Lake could be expected to be unpolluted, and this observation is confirmed by comparing the examined metal concentration values to SQGs values (Figs. 3, 4, 5, 6, and 7), indicating a rather low ecological risk for organisms. However, anthropogenic influences affecting the Wigry Lake environment are rather undeniable and result in higher values of concentration of metals in lake sediments. Previous studies (Kostka et al. 2008; Kostka 2009) employing the so-called multicriteria evaluation (a GIS tool using correlations between spatial data in interpolation procedures) revealed, for example, negative correlations between the values of the concentration of metals and the distance from roads, land, and inhabited areas, which may indicate an anthropogenic source of examined elements. Positive correlations between the values of the concentration of metals and the distance from river tributaries may indicate both natural and anthropogenic sources of the examined elements, as they may originate both from natural leaching of weathering postglacial rocks covering the catchment area and from human activity leading to water system contamination.
The attempts to assess the degree of contamination of Wigry Lake sediments highlighted the significance of proper and careful determination of the geochemical background, raised previously by other authors (e.g., Matschullat et al. 2000; Reimann et al. 2005; Gałuszka 2007; Gałuszka and Migaszewski 2011; Dung et al. 2013; Bábek et al. 2015; Matys Grygar and Popelka 2016; Xu et al. 2017). It is interesting to note that the introduction of the GB3 background values to calculate the pollution indices resulted in meaningfully distinct assessment results. This is particularly evident in the case of lead, in the case of which Cf values significantly decreased when calculated according to GB3 (Table 6). As a result, Cd and PLI indices (Fig. 9), affected mostly by Pb, were also considerably reduced. As mentioned above, lead is geochemically distinct from other examined elements, which implies further observations, e.g., Pb did not correlate with other metals or depth, and relationships with other spatial factors were also weak (Kostka 2009). It was concluded that this element may originate from atmospheric precipitation or from the use of agricultural fertilizers, and the contribution of the anthropogenic sources of this element in sediments seems to be the most significant in comparison to the other examined metals (Kostka 2009; Aleksander-Kwaterczak and Kostka 2011). This conclusion is supported by an assessment performed according to SQGs (Smith et al. 1996), indicating that Pb content in most samples of Wigry Lake sediments falls within the range of concentrations occasionally associated with adverse biological effects (TEL < [Pb] < PEL) (Fig. 6), while concentrations of other examined metals were below the TEL value in most or even all samples (Figs. 3, 4, 5, and 7). However, as was mentioned above, SQGs do not consider physiochemical properties of deposits or in situ conditions, which affect the bioavailability of pollutants. In the case of Pb, as a poorly mobile element which is easily immobilized in carbonates (Hamilton-Taylor and Davison 1995; Bojakowska et al. 2014), bioavailability is rather low. Nearly half of sediment samples (43%) exceeded the GB3 value for lead, while, for other metals, relevant percentages were below 11%. However, it should be remembered that the structure of the sampling set in the case of Pb was distinct from other metals. For Cd, Cr, Cu, and Zn, the most abundant data sets were carbonate gyttjas, and the number of samples with the highest levels of the concentration of metals (fluvial-lacustrine sediment) was relatively low. In the case of lead, the most abundant data set was for lacustrine chalk (Table 3), and this sediment type was characterized by relatively high levels of Pb concentration. Most samples in which the GB3 value for Pb (70.9 mg kg−1) was exceeded were identified as lacustrine chalk or fluvial-lacustrine sediment (Fig. 8). Taking into account all of the above, it can be assumed that although the levels of concentration of Pb in Wigry Lake sediments are undeniably high, the ecological risk connected with this element is rather low. The anthropogenic source of lead is not known, but most probably, it originates from atmospheric precipitation and/or from agriculture.
5 Summary and conclusions
Wigry Lake is unique in terms of sediment research, both in Poland and in the world, because, over many years of interdisciplinary investigation, about 1200 bottom sediment samples were collected, of which nearly 500 were tested for the concentration of metals. Meanwhile, most similar studies often rely on a much smaller number of samples, usually taken from the deepest parts of the lake. Attention is not always paid to spatial factors that may affect the distribution of metals, the type of sediment, or sedimentation facies from which the samples are taken. The studies carried out on the Wigry Lake have demonstrated that these issues are extremely important. A uniquely large collection of samples enabled us to “capture” the vast majority of geochemical anomalies and pay attention to sedimentation conditions and their effect on metal accumulation.
Five types of geochemical backgrounds and several pollution indices used to assess the chemoecological state of Wigry Lake sediments revealed the importance of properly defining any reference values. Each environmental case should be treated individually, and reference to any regional or global threshold values is of doubtful usefulness for many reasons. Our research revealed that even very locally calculated background values may generate extremely diversified results, indicating that the Wigry Lake environment is uncontaminated to extremely polluted. Finally, the lake was assessed as slightly contaminated in terms of Cd, Cr, Cu, and Zn, while the levels of the concentration of Pb in the sediments seem to be more, but still not seriously, disturbing.
Availability of data and material
We declare that all data and materials, as well as software application, we published claim and comply with field standards.
Code availability
Not applicable.
References
Abdel Gawad SS (2018) Concentrations of heavy metals in water, sediment and mollusc gastropod, Lanistes carinatus from Lake Manzala, Egypt. Egypt J Aquat Res 44(2):77–82
Aleksander-Kwaterczak U, Kostka A (2011) Lead in the environment of Lake Wigry (NE Poland). Limnol Rev 11(2):59–68
Aleksander-Kwaterczak U, Plenzler D (2019) Contamination of small urban watercourses on the example of a stream in Krakow (Poland). Environ Earth Sci 78:530
Aleksander-Kwaterczak U, Prosowicz D (2007) Distribution of Cd, and Pb in the lake sediments cores from the Hańczańska Bay (Wigry Lake, NE Poland). Limnol Rev 7(4):215–219
Aleksander-Kwaterczak U, Zdechlik R (2016) Hydrogeochemical characteristics of interstitial water and overlying water in the lacustrine environment. Environ Earth Sci 75:1352
Aleksander-Kwaterczak U, Prosowicz D, Rutkowski J, Szczepańska J (2009) Changes of selected micro-pollution concentrations in the long carbonate sediment cores of the southern part of Wigry Lake (NE Poland). Pol J Environ Stud 18(2B):51–55
Bábek O, Matys Grygar T, Faměra M, Hron K, Nováková T, Sedláček J (2015) Geochemical background in polluted river sediments: how to separate the effects of sediment provenance and grain size with statistical rigour? Catena 135:240–253
Boës X, Rydberg J, Martinez-Cortizas A, Bindler R, Renberg I (2011) Evaluation of conservative lithogenic elements (Ti, Zr, Al, and Rb) to study anthropogenic element enrichments in lake sediments. J Paleolimnol 46:75–87
Bojakowska I, Krasuska J, Retka J, Wiłkomirski B (2014) Lead concentration and the content of selected macroelements in lake sediments in Poland. J Elem 3:627–636
Boyle JF (2001) Inorganic geochemical methods in paleolimnology. In: Last WM, Smol JP (eds) Tracking Environmental Change Using Lake Sediments, vol 2, Physical and Geochemical Methods, Springer Netherlands, pp 83–141
Burrough PA, McDonnell RA (1998) Principles of geographical information systems. Oxford University Press, Oxford
Burton ED, Phillips IR, Hawker DW (2005) Geochemical partitioning of copper, lead, and zinc in benthic, estuarine sediment profiles. J Environ Qual 34(1):263–273
Chen M, Ma LQ, Hoogeweg CG, Harris WG (2001) Arsenic background concentrations in Florida, U.S.A. surface soils: determination and interpretation. Environ Forensic 2(2):117–126
Cobelo-García A, Prego R (2003) Heavy metal sedimentary record in a Galician Ria (NW Spain): background values and recent contamination. Mar Pollut Bull 46(10):1253–1262
Coquery M, Welbourn PM (1995) The relationship between metal concentration and organic matter in sediments and metal concentration in the aquatic macrophyte Eriocaulon septangulare. Water Res 29(9):2094–2102
Dai L, Wang L, Li L, Liang T, Zhang Y, Ma C, **ng B (2018) Multivariate geostatistical analysis and source identification of heavy metals in the sediment of Poyang Lake in China. Sci Total Environ 621:1433–1444
Dung TTT, Cappuyns V, Swennen R, Phung NK (2013) From geochemical background determination to pollution assessment of heavy metals in sediments and soils. Rev Environ Sci Biotechnol 12(4):335–353
El Bilali L, Rasmussen PE, Hall GEM, Fortin D (2002) Role of sediments composition in trace metal distribution in lake sediments. Appl Geochem 17(9):1171–1181
El-Kady AA, Wade TL, Sweet ST, Klein AG (2019) Spatial distribution and ecological risk assessment of trace metals in surface sediments of Lake Qaroun, Egypt. Environ Monit Assess 191:413
EU WFD (2011) Technical guidance for deriving environmental quality standards. Common implementation strategy for the Water Framework Directive (2000/60/EC) Guidance Document No. 27, European Commission, Brussels, Belgium
Gałuszka A (2007) Different approaches in using and understanding the term geochemical background – practical implications for environmental studies. Pol J Environ Stud 16(3):389–395
Gałuszka A, Migaszewski ZM (2011) Geochemical background – an environmental perspective. Mineralogia 42(1):7–17
Gan FF, Koehler KJ (1990) Goodness-of-fit tests based on P-P probability plots. Technometrics 32(3):289–303
GUS (2019) Population. Size and structure and vital statistics in Poland by territorial division in 2019. As of 30th June. https://stat.gov.pl (accessed: June 2020)
Håkanson L (1980) An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res 14:975–1001
Hamilton-Taylor J, Davison W (1995) Redox-driven cycling of trace elements in lakes. In: Lerman A, Imboden D, Gat J (eds) Physics and Chemistry of Lakes. Springer-Verlag, Berlin, pp 217–263
Helios-Rybicka E, Kostka A (2007) Distribution of Fe, Mn and Zn in bottom sediments of Wigierskie Basin – Wigry Lake, (NE Poland). Pol J Environ Stud 16(3B):162–167
Islam MS, Hossain MB, Matin A, Sarker MSI (2018) Assessment of heavy metal pollution, distribution and source apportionment in the sediment from Feni River estuary, Bangladesh. Chemosphere 202:25–32
Juśkiewicz WW, Marszelewski W, Tylmann W (2015) Differentiation of the concentration of heavy metals and persistent organic pollutants in lake sediments depending on the catchment management (Lake Gopło case study). Bull Geogr Phys Geogr Ser 8:71–80
Karasiewicz TM, Hulisz P, Noryśkiewicz AM, Stachowicz-Rybka R (2019) Post-glacial environmental history in NE Poland based on sedimentary records from the Dobrzyń Lakeland. Quat Int 501(A):193–207
Kirby J, Maher W, Krikowa F (2001) Selenium, cadmium, copper, and zinc concentrations in sediments and mullet (Mugil cephalus) from the Southern Basin of Lake Macquarie, NSW, Australia. Arch Environ Contam Toxicol 40(2):246–256
Kostka A (2009) Assessment of spatial variability of trace metals in bottom sediments of Lake Wigry using GIS techniques. PhD thesis, AGH University of Science and Technology, Krakow, Poland (in Polish)
Kostka A, Leśniak A (2020) Spatial and geochemical aspects of heavy metal distribution in lacustrine sediments, using the example of Lake Wigry (Poland). Chemosphere 240:124879
Kostka A, Prosowicz D, Helios-Rybicka E (2008) Use of GIS for spatial analysis of heavy metals distribution in the bottom sediments of Wigry Lake (NE Poland). Pol J Environ Stud 17(3A):307–312
Król K (1998) Preliminary results of lacustrine chalk survey from Lake Wigry. Sprawozdania z Czynności i Posiedzeń PAU LXI:112–114 (in Polish)
Król K (2017) Obituary – Prof. Jacek Rutkowski (1934–2016). Geochronometria 44:I
Kulbat E, Sokołowska A (2019) Methods of assessment of metal contamination in bottom sediments (case study: Straszyn Lake, Poland). Arch Environ Contam Toxicol 77(4):605–618
Kupryjanowicz M (2007) Postglacial development of vegetation in the vicinity of the Wigry Lake. Geochronometria 27:53–66
Kuriata-Potasznik A, Szymczyk S, Skwierawski A, Glińska-Lewczuk K, Cymes I (2016) Heavy metal contamination in the surface layer of bottom sediments in a flow-through lake: a case study of Lake Symsar in Northern Poland. Water 8:358
Lin Q, Liu E, Zhang E, Li K, Shen J (2016) Spatial distribution, contamination and ecological risk assessment of heavy metals in surface sediments of Erhai Lake, a large eutrophic plateau lake in southwest China. Catena 145:193–203
Lis J, Pasieczna A (1995) Geochemical Atlas of Poland on a scale 1:2 500 000. Państwowy Instytut Geologiczny, Warszawa (in Polish)
MacDonald DD, Ingersoll CG, Berger TA (2000) Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Arch Environ Contam Toxicol 39(1):20–31
Matschullat J, Ottenstein R, Reimann C (2000) Geochemical background – can we calculate it? Environ Geol 39(9):990–1000
Matys Grygar TM, Popelka J (2016) Revisiting geochemical methods of distinguishing natural concentrations and pollution by risk elements in fluvial sediments. J Geochem Explor 170:39–57
McKinley JM, Hron K, Grunsky EC, Reimann C, de Caritat P, Filzmoser P, van den Boogaart KG, Tolosana-Delgado R (2016) The single component geochemical map: fact or fiction? J Geochem Explor 162:16–28
Migaszewski ZM, Gałuszka A, Pasławski P (2003) Baseline versus background concentrations of trace elements in sediments of Lake Wigry, NE Poland. Limnol Rev 3:165–172
Milošković A, Branković S, Simić V, Kovačević S, Cirković M, Manojlović D (2013) The accumulation and distribution of metals in water, sediment, aquatic macrophytes and fishes of the Gruža Reservoir, Serbia. Bull Environ Contam Toxicol 90:563–569
Müller G (1969) Index of geoaccumulation in sediments of the Rhine River. Geojournal 2:108–118
Nguyen HL, Leermakers M, Osán J, Tӧrӧk S, Baeyens W (2005) Heavy metals in Lake Balaton: water column, suspended matter, sediment and biota. Sci Total Environ 340(1–3):213–230
Nobi EP, Dilipan E, Thangaradjou T, Sivakumar K, Kannan L (2010) Geochemical and geo-statistical assessment of heavy metal concentration in the sediments of different coastal ecosystems of Andaman Islands, India. Estuar Coast Shelf Sci 87(2):253–264
Ouchir N, Aissa LB, Boughdiri M, Aydi A (2016) Assessment of heavy metal contamination status in sediments and identification of pollution source in Ichkeul Lake and rivers ecosystem, northern Tunisia. Arab J Geosci 9:593
Pawlyta J, Pazdur A, Piotrowska N, Poręba G, Sikorski J, Szczepanek M, Król K, Rutkowski J, Hałas S (2004) Isotopic investigation of uppermost sediments from Wigry Lake (NE Poland) and its environment. Geochronometria 23:71–78
Pebesma EJ, de Jong K, Briggs DJ (2007) Visualising uncertain spatial and spatiotemporal data under different scenarios: an air quality example. Int J Geogr Inf Sci 21:515–527
Piotrowska N, Hajdas I, Bonani G (2007) Construction of the calendar timescale for lake Wigry (NE Poland) sediments on the basis of radiocarbon dating. Radiocarbon 49(2):1133–1143
Pradit S, Wattayakorn G, Angsupanich S, Baeyens W, Leemakers M (2010) Distribution of trace elements in sediments and biota of Songkhla Lake, Southern Thailand. Water Air Soil Pollut 206(1–4):155–174
Prosowicz D, Helios-Rybicka E (2002) Trace metals in recent bottom sediments of Lake Wigry (Bryzgiel Basin). Limnol Rev 2:323–332
Ramsey MH (1993) Sampling and analytical quality control (SAX) for improved error estimation in the measurement of Pb in the environment using robust analysis of variance. Appl Geochem 8(2):149–153
Ramsey MH, Thompson M, Hale M (1992) Objective evaluation of precision requirements for geochemical analysis using robust analysis of variance. J Geochem Explor 44(1–3):23–36
Rashki Ghaleno O, Sayadi MH, Rezaei MR (2015) Potential ecological risk assessment of heavy metals in sediments of water reservoir case study: Chah Nimeh of Sistan. Proc Int Acad Ecol Environ Sci 5(4):89–96
Reimann C, de Caritat P (2000) Intrinsic flaws of element enrichment factors (EFs) in environmental geochemistry. Environ Sci Technol 34:5084–5091
Reimann C, de Caritat P (2005) Distinguishing between natural and anthropogenic sources for elements in the environment: regional geochemical surveys versus enrichment factors. Sci Total Environ 337:91–107
Reimann C, Filzmoser P, Garret RG (2005) Background and threshold: critical comparison of methods of determination. Sci Total Environ 346(1–3):1–16
Rognerud S, Hongve D, Fjeld E, Ottesen RT (2000) Trace metal concentrations in lake and overbank sediments in southern Norway. Environ Geol 39(7):723–732
Rutkowski J (2004) Sediments of Wigry Lake. Rocznik Augustowsko-Suwalski IX:19–37 (in Polish)
Rutkowski J (2007) Simple gravity sampler for taking cores from lake sediments. In: Kubiak J, Bajkiewicz-Grabowska E (eds) Anthropogenic and natural transformation of lakes, vol 1, Szczecin, pp 108–109
Rutkowski J, Król K, Krzysztofiak L, Prosowicz D (2002a) Recent sediments of the Wigry Lake (Bryzgiel Basin). Limnol Rev 2:353–362
Rutkowski J, Rudowski S, Pietsch K, Król K, Krzysztofiak L (2002b) Sediments of Lake Wigry (NE Poland) in the light of high-resolution seismic (seismoacoustic) survey. Limnol Rev 2:363–371
Rutkowski J, Król K, Krzysztofiak L, Prosowicz D (2003) Recent sediments of the Wigry Lake (Szyja Basin). Limnol Rev 3:197–203
Rutkowski J, Pietsch K, Król K, Rudowski S, Krzysztofiak L (2005) High-resolution seismic survey in the Wigry Lake (NE Poland). Peribalticum 9:147–162
Rutkowski J, Król K, Szczepańska J (2007) Lithology of the profundal sediments in Słupiańska Bay (Wigry Lake, NE Poland) – introduction to interdisciplinary study. Geochronometria 27:47–52
Rutkowski J, Prosowicz D, Aleksander-Kwaterczak U, Krzysztofiak L (2008) Profundal sediments of Wigry Lake (NE Poland). In: Bajkiewicz-Grabowska E, Borowiak D (eds) Anthropogenic and natural transformation of lakes, vol 2, Gdańsk, pp 171–174
Rutkowski J, Prosowicz D, Aleksander-Kwaterczak U, Krzysztofiak L (2009) Sediments of Wigry Lake. In: Rutkowski J, Krzysztofiak L (eds) Wigry Lake: history of the lake in the light of geological and paleoecological studies, pp 83–109
Salam MA, Paul SC, Shaari FI, Rak AE, Ahmad RB, Kadir WR (2019) Geostatistical distribution and contamination status of heavy metals in the sediment of Perak River, Malaysia. Hydrology 6(30)
Salomons W, Förstner U (1984) Metals in the Hydrocycle. Springer-Verlag, Berlin
Selvam AP, Priya SL, Banerjee K, Hariharan G, Purvaja R, Ramesh R (2012) Heavy metal assessment using geochemical and statistical tools in the surface sediments of Vembanad Lake, Southwest Coast of India. Environ Monit Assess 184:5899–5915
Selvaraj K, Ram Mohan V, Szefer P (2004) Evaluation of metal contamination in coastal sediments of the Bay of Bengal, India: geochemical and statistical approaches. Mar Pollut Bull 49(3):174–185
Simpson SL, Batley GB, Chariton AA (2013) Revision of the ANZECC/ARMCANZ Sediment Quality Guidelines. CSIRO Land and Water Science Report 08/07. CSIRO, Canberra, Australia
Singh KP, Mohan D, Singh VK, Malik A (2005) Studies on distribution and fractionation of heavy metals in Gomti River sediments – a tributary of the Ganges, India. J Hydrol 312(1–4):14–27
Smith SL, MacDonald DD, Keenleyside KA, Ingersoll CG, Field LJ (1996) A preliminary evaluation of sediment quality assessment values for freshwater ecosystems. J Great Lakes Res 22(3):624–638
Soliman NF, Nasr SM, Okbah MA (2015) Potential ecological risk of heavy metals in sediments from the Mediterranean coast, Egypt. J Environ Health Sci Eng 13:70
Superville PJ, Prygiel E, Magnier A, Lesven L, Gao Y, Baeyens W, Ouddane B, Dumoulin D, Billon G (2014) Daily variations of Zn and Pb concentrations in the Deûle River in relation to the resuspension of heavily polluted sediments. Sci Total Environ 470–471(1):600–607
Sutherland R (2000) Bed sediment-associated trace metals in an urban stream, Oahu, Hawaii. Environ Geol 39(6):611–627
Szyczewski P, Siepak J, Niedzielski P, Sobczyński T (2009) Research on heavy metals in Poland. Pol J Environ Stud 18(5):755–768
Taylor SR, McLennan SM (1995) The geochemical evolution of the continental crust. Rev Geophys 33(2):241–265
Thin MM, Setti M, Sacchi E, Re V, Riccardi MP, Allais E (2020) Mineralogical and geochemical characterisation of alkaline lake sediments to trace origin, depositional processes, and anthropogenic impacts: Inle Lake (Southern Shan State, Myanmar). Environ Earth Sci 79:166
Tomlinson DL, Wilson JG, Harris CR, Jeffery DW (1980) Problems in the assessment of heavy-metals levels in estuaries and the formation of a pollution index. Helgol Meeresunters 33(1–4):566–575
Turekian KK, Wedephol KH (1961) Distribution of the elements in some major units of the earth’s crust. Geol Soc Am Bull 72:175–192
Turner SD, Rose NL, Goldsmith BN, Bearcock JM, Scheib C, Yang H (2017) Using public participation to sample trace metals in lake surface sediments: the OPAL Metals Survey. Environ Monit Assess 189:241
Tylmann W, Łysek K, Kinder M, Pempkowiak J (2011) Regional pattern of heavy metal content in lake sediments in Northeastern Poland. Water Air Soil Pollut 216(1–4):217–228
Tylmann W, Głowacka P, Szczerba A (2017) Tracking climate signals in varved lake sediments: research strategy and key sites for comprehensive process studies in the Masurian Lakeland. Limnol Rev 17(3):159–166
Varol M (2011) Assessment of heavy metal contamination in sediments of the Tigris River (Turkey) using pollution indices and multivariate statistical techniques. J Hazard Mater 195:355–364
Wang Y, Hu J, **ong K, Huang X, Duan S (2012) Distribution of heavy metals in core sediments from Baihua Lake. Procedia Environ Sci 16:51–58
Wardas M, Aleksander-Kwaterczak U, Jusik S, Hryc B, Zgoła T, Sztuka M, Kaczmarska M, Mazurek M (2010) An attempt to assess the impact of anthropopressure on the ecological state of urbanized watercourses of Krakow Conurbation and the difficulties encountered. J Elem 15(4):725–743
Wilk-Woźniak E, Pociecha A, Ciszewski D, Aleksander-Kwaterczak U, Walusiak E (2011) Phyto- and zooplankton in fishponds contaminated with heavy metal runoff from a lead-zinc mine. Oceanol Hydrobiol St 40(4):77–85
Xu F, Liu Z, Yuan S, Zhang X, Sun Z, Xu F, Jiang Z, Li A, Yin X (2017) Environmental background values of trace elements in sediments from the Jiaozhou Bay catchment, Qingdao, China. Mar Pollut Bull 121(1–2):367–371
Yao Z, Gao P (2007) Heavy metal research in lacustrine sediment: a review. Chin J Oceanol Limnol 25(3):444–454
Yi Y, Yang Z, Zhang S (2011) Ecological risk assessment of heavy metals in sediment and human health risk assessment of heavy metals in fishes in the middle and lower reaches of the Yangtze River basin. Environ Pollut 159(10):2575–2585
Zawisza E, Szeroczyńska K (2007) The development history of Wigry Lake as shown by subfossil Cladocera. Geochronometria 27:67–74
Zdanowski B (2003) Precipitation of phosphorus in the zone of river and lake water mixing: R. Hańcza and Lake Wigry (North-East Poland). Pol J Ecol 51(2):143–154
Zhang J, Li ZH, Chen J, Wang M, Tao R, Liu D (2014) Assessment of heavy metal contamination status in sediments and identification of pollution source in Daye Lake, Central China. Environ Earth Sci 72:1279–1288
Zhang H, Jiang Y, Ding M, **e Z (2017) Level, source identification, and risk analysis of heavy metal in surface sediments from river-lake ecosystems in the Poyang Lake, China. Environ Sci Pollut Res Int 24(27):21902–21916
Acknowledgements
The results presented in this work are the effect of many years of research carried out thanks to the passion of one man–Professor Jacek Rutkowski (Król 2017) and the involvement of his friends and collaborators. We would like to thank all of them.
Funding
The study was financially supported by the Faculty of Geology, Geophysics and Environmental Protection at the AGH University of Science and Technology in Cracow, Poland (no. 16.16.140.315).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Urszula Aleksander-Kwaterczak and Anna Kostka. Cartographic and statistical analysis was performed by Andrzej Leśniak and Anna Kostka. The first draft of the manuscript was written by Urszula Aleksander-Kwaterczak and all authors contributed in revision and creation of final versions of the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
Not applicable
Informed consent
Not applicable
Additional information
Responsible editor: Tomas Matys Grygar
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aleksander-Kwaterczak, U., Kostka, A. & Leśniak, A. Multiparameter assessment of select metal distribution in lacustrine sediments. J Soils Sediments 21, 512–529 (2021). https://doi.org/10.1007/s11368-020-02732-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-020-02732-x