Abstract
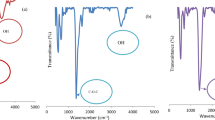
In this research work, poly(o-phenylenediamine) was incorporated into the hydrous zirconium oxide matrix to form poly(o-phenylenediamine)/hydrous zirconium oxide composite which is used for the removal of Cd(II) from aqueous solution. The characterization of the material was done based on FTIR, XRD, SEM, and TGA-DTA. The effects of contact time, pH, adsorbent dose, and initial concentration of Cd(II) on the removal of Cd(II) were studied by performing 29 sets of sorption runs using Box–Behnken design combined with response surface methodology (RSM). Various isotherm models were tested to describe the adsorption equilibrium. The adsorption equilibrium data fitted well with Freundlich isotherm model. The maximum adsorption capacity of 66.66 mg g−1 was obtained from Langmuir isotherm. The pseudo-second-order kinetic model described the adsorption kinetics more accurately. Diffusion-based kinetics such as intraparticle diffusion and Bangham’s model suggested that both film and intraparticle pore diffusion were involved in the adsorption process. The Elovich model pointed towards the chemisorption. The investigation of desorption and regeneration suggested that the material can be used as an effective sorbent for removal of Cd(II) from aqueous system.
















Similar content being viewed by others
References
Ahamd AL, Shahbuddin MMH, Ooi BS, Kusumastuti A (2016) Cadmium removal from aqueous solution by emulsion liquid membrane (ELM): influence of emulsion formulation on cadmium removal and emulsion swelling. Desalin Water Treat 57:28274–28283
Ahsainea HA, Zbairb M, El Haoutia R (2017) Mesoporous treated sewage sludge as outstanding low-cost adsorbent for cadmium removal. Desalin Water Treat 85:330–338
Ali AAM, Zaki MI (1998) Fourier-transform laser Raman spectroscopy of adsorbed pyridine and nature of acid sites on calcined phosphate/Zr (OH)4. Colloids Surf A Physicochem Eng Asp 139:81–89
Al-Malack MH, Al-Attas OG, Basaleh AA (2017) Competitive adsorption of Pb2+ and Cd2+ onto activated carbon produced from municipal organic solid waste. Desalin Water Treat 60:310–318
Anwar J, Shafique U, Zaman WU, Salman M, Dar A, Anwar S (2010) Removal of Pb (II) and Cd (II) from water by adsorption on peels of banana. Bioresour Technol 101:1752–1755
Azouaou N, Mokaddem H (2010) Adsorption of cadmium from aqueous solution onto untreated coffee grounds: equilibrium, kinetics and thermodynamics. J Hazard Mater 184:126–134
Badruddoza AZM, Shawon ZBZ, Daniel WJD, Hidajat K, Uddin MS (2013) Fe3O4/cyclodextrin polymer nanocomposites for selective heavy metals removal from industrial wastewater. Carbohydr Polym 91:322–332
Bandari F, Safa F, Shariati SH (2015) Application of response surface methodology for optimization of adsorptive removal of Eriochrome Black T using magnetic multi-wall carbon nanotube nanocomposite. Arab J Sci Eng 40:3363–3372
Bernard A (2008) Cadmium and its adverse effects on human health. Indian J Med Res 128:557–564
Boudrahem F, Soualah A, Aissani-Benissad F (2011) Pb (II) and Cd (II) removal from aqueous solution using activated carbon developed from coffee residue activated with phosphonic acid and zinc chloride. J Chem Eng Data 56:1946–1955
Cadaval TRS Jr, Dotto GL, Seus ER, Mirlean N, Pinto LAA (2016) Vanadium removal from aqueous solutions by adsorption onto chitosan. Desalin Water Treat 57:16583–16591
Candioti LV, Zan MMD, Camara MS, Goicoechea HC (2014) Experimental design and multiple response optimization using the desirability function in analytical methods development. Talanta 124:123–138
Cerrahoglu F, Kayan A, Bingol A (2017) New inorganic-organic hybrid materials and their oxides for removal of heavy metal ions: response surface methodology approach. J Inorg Organomet Polym 27:427–435
Chen G, Zeng G, Tang L, Du C, Jiang X, Huang G, Liu H, Shen G (2008) Cadmium removal from simulated wastewater to biomass byproduct of Lentinusedodes. Bioresour Technol 99:7034–7040
Derringer G, Suich R (1980) Simultaneous optimization of several response variables. J Qual Technol 12:214–219
Dey RK, Patnaik T, Singh VK, Swain SK, de Melo MA, Airoldi C (2010) Al-centered functionalized inorganic–organic hybrid sorbent containing N and S donor atoms for effective removal of cadmium. Solid State Sci 12:440–447
Ding Y, **g D, Gong H, Zhou L, Yong X (2012) Biosorption of aquatic cadmium (II) by unmodified rice straw. Bioresour Technol 114:20–25
Directive 2006/11/EC of the European Parliament and of Council of 15 February 2006 on pollution caused by certain dangerous substances discharged into the aquatic environment of the community
Dou X, Mohan D, Pittman CU Jr, Yang S (2012) Remediating fluoride from water using hydrous zirconium oxide. Chem Eng J 198-199:236–245
Dubey A, Mishra A, Singhal S (2014) Application of dried plant biomass as novel adsorbent for removal of cadmium from aqueous solution. Int J Environ Sci Technol 11:1043–1050
Farasati M, Haghighi S, Boroun S (2016) Cd removal from aqueous solution using agricultural wastes. Desalin Water Treat 57:1116–11172
Garg U, Kaur MP, Jawa GK, Sud D, Garg VK (2008) Removal of cadmium (II) from aqueous solutions by adsorption on agricultural wastes biomass. J Hazard Mater 154:1149–1157
Gavris G, Caraban A, Stanarel O, Badea GE (2013) Cadmium ions recovery by chemicals precipitation method from residual solutions of galvanic plating, J. Sustainable Energy 4:1–5
Gaya UI, Otene E, Abdullah AH (2015) Adsorption of aqueous Cd (II) and Pb (II) on activated carbon nanopores prepared by chemical activation of doum palm shell. Springer Plus 4:458. https://doi.org/10.1186/s40064-015-1256-4
Georgescu I, Mureseanu M, Carja G, Hulea V (2013) Adsorptive removal of cadmium and copper from water by mesoporous silica functionalized with N-( aminothioxomethyl)-2-thiophen carboxamide. J Environ Eng 139:1285–1296
Gupta VK, Singh P, Rahman N (2005) Synthesis, characterization and analytical application of zirconium(IV) selenoiodate, a new cation exchanger. Anal Bioanal Chem 381:471–476
Hasanzadeh R, Moghadam PN, Bahri-Laleh N, Sillanpaa M (2017) Effective removal of toxic metal ions from aqueous solutions: 2-bifunctional magnetic nanocomposite base on novel reactive PGMA-MAn copolymer @ Fe3O4 nanocomposites. J Colloid Interface Sci 490:727–746
Heffron J, Marhefke M, Mayer BK (2016) Removal of trace metal concentration from potable water by electrocoagulation. Sci Rep 6:28478. https://doi.org/10.1038/Sreo28478
Hegazi HA (2013) Removal of heavy metals from wastewater using agricultural and industrial wastes as adsorbents. HBRC J 9:276–282
Hema M, Srinivasan K (2011) Removal of cadmium (II) from wastewater using activated carbon prepared from agro industrial by products. J Environ Sci Eng 53:387–396
Hossain MA, Ngo HH, Guo W (2013) Introductory of Microsoft Excel SOLVER function-spreadsheet method for isotherm and kinetics modelling of metal biosorption in water and wastewater. J Water Sustainability 3:223–227
Huang M-R, Li X-G, Yang Y (2001) Oxidative polymerization of o-phenylenediamine and pyrimidylamine. Polym Degrad Stab 71:31–38
Husein DZ, Aazam E, Battia M (2017) Adsorption of cadmium (II) onto watermelon rind under microwave radiation and application into surface water from Jeddah, Saudi Arabia. Arab J Sci Eng 42:2403–2415
Ichinohe D, Saitoh N, Kise H (1998) Oxidative polymerization of phenylenediamines in reversed micelles. Macromol Chem Phys 199:1241–1245
Inyinbor AA, Adekola FA, Olatunji GA (2016) Kinetics, isotherms and thermodynamic modeling of liquid phase adsorption of rhodamine B dye onto Raphia hookerie fruit epicarp. Water Resour Ind 15:14–27
IS 10500 (1992) Drinking water specification (Reaffirmed 1993); http://www.hppcb.nic.in/ElAsorang/spec.pdf; 8.9.2007
Jain M, Garg VK, Kadirvelue K (2011) Investigation of Cr (VI) adsorption onto chemically treated Helianthus annuus: optimization using response surface methodology. Bioresour Technol 102:600–605
Javadian H, Ghorbani F, Tayebi H, Hosseini SM (2015) Study of the adsorption of cd (II) from aqueous solution using zeolite-based geopolymer, synthesized from coal fly ash; kinetic, isotherm and thermodynamic studies. Arab J Chem 8:837–849
Jimoh AA, Adebayo GB, Otum KO, Ajeboy AT, Bale AT, Jamiu W, Alao FO (2015) Sorption study of Cd (II) from aqueous solution using activated carbon prepared from Vitellaria paradoxa shell. J Bioremed Biodeg 6:288. https://doi.org/10.4172/2155-6199.1000288
Kakaei A, Kazemeini M (2016) Removal of Cd (II) in water sample using modified magnetic iron oxide nanoparticles. Iranian J Toxicol 10:9–14
Karthik R, Meenakshi S (2015) Removal of Pb(II) and Cd(II) ions from aqueous solution using polyaniline grafted chitosan. Chem Eng J 263:168–177
Karunanayake AJ, Todd OA, Crowley M, Ricchetti L, Pittman CU Jr, Anderson R, Mohan D (2018) Lead and cadmium remediation using magnetized and nonmagnetized biochar from Douglas fir. Chem Eng J 331:480–491
Kayan A, Arican MO, Boz Y, Ay U, Bozbas SK (2014) Novel tyrosine-containing inorganic-organic hybrid adsorbent in removal of heavy metal ions. J Environ Chem Eng 2:935–942
Kim E-J, Lee C-S, Chang Y-Y, Chang Y-S (2013) Hierarchically structured manganese oxide-coated maneticnano composites for the efficient removal of heavy metal ions from aqueous systems. ACS Appl Mater Interfaces 5:9628–9634
Kobya M, Demirbas E, Senturk E, Ince M (2005) Adsorption of heavy metal ions from aqueous solutions by activated carbon prepared from apricot stone. Bioresour Technol 96:1518–1521
Kong Q, **e B, Preis S, Hu Y, Wu H, Wei C (2018) Adsorption of Cd2+ by ion –imprinted thiol-functionalized polymer in competition with heavy metal ions and organic acids. RSC Adv 8:8950–8960
Krika F, Azzouz N, Ncibi MC (2016) Adsorptive removal of cadmium from aqueous solution by cork biomass: equilibrium, dynamic and thermodynamic studies. Arab J Chem 9:51077–51083
Lin X, Burns RC, Lawrance GA (2005) Heavy metal ions wastewater: the effect of electrolyte composition on the precipitation of Cd (II) using lime and magnesia. Water Air Soil Pollut 165:131–152
Liu F, ** Y, Liao H, Cai L, Tong M, Hou Y (2013) Facile self-assembly synthesis of titanate / Fe3O4 nanocomposites for the efficient removal of Pb2+from aqueous systems. J Mater Chem A 1:805–813
Liu Z, Li X, Zhan P, Hu F and Ye X (2018) Removal of cadmium and copper from water by magnetic adsorbent of PFM: adsorption performance and microstructural morphology, Sep Purif Technol, (in press). https://doi.org/10.1016/j.seppur.2018.06.007
Mahmoud ME, Abdou AE, Ahmad SB (2016) Conversion of waste Styrofoam into engineered adsorbents for efficient removal of cadmium, lead and mercury from water. ACS Sustain Chem Eng 4:819–827
Maleki A, Mahvi AH, Zazouli MA, Izanloo H, Barati AH (2011) Aqueous cadmium removal by adsorption on barley hull and barley hull ash. Asian J Chem 23:1373–1376
Markandeya, Shukla SP, Kisku GC (2015) Linear and nonlinear kinetic modelling for adsorption of disperse dye in batch process. Res J Environ Toxicol 9:320–331
Mehdinia A, Sehegfti S, Shemirani F (2015) Removal of lead (II), copper (II) and zinc (II) ions from aqueous solutions using magnetic amine-functionalized mesoporous silica nanocomposites. J Braz Chem Soc 26:2249–2257
Milonjic SK (2007) A consideration of the correct calculation of thermodynamic parameters of adsorption. J Serb Chem Soc 72:1363–1367
Mishra SP, Singh VK, Tiwari D (1996a) Efficient removal of zinc ions from aqueous solution by hydrous zirconium oxide. J Radioanal Nucl Chem 210:207–217
Mishra SP, Singh VK, Tiwari D (1996b) Radiotracer technique in adsorption study: part XIV. Efficient removal of mercury from aqueous solutions by hydrous zirconium oxide. Appl Radiat Isot 47:15–21
Mishra SP, Singh VK, Tiwari D (1997) Inorganic particulates in removal of heavy metal ions. Efficient removal of cadmium ions from aqueous solution by hydrous zirconium oxide. Radiochim Acta 76:97–101
Mohammed RR (2012) Removal of heavy metals from wastewater using black teawaste. Arab J Sci Eng 37:1505–1520
Mohanapriya T, Kumar PE (2016) Removal of cadmium (II) from aqueous solution on activated carbon prepared from Typha angustata L: equilibrium and kinetic studies. Int J Sci Res Publ 6:192–203
Mopoung R, Kengkhetkit N (2016) Lead and cadmium removal efficiency from aqueous solution by NaOH treated pineapple waste. Int J Appl Chem 12:23–35
Moubarik A, Grimi N (2015) Valorization of olive stone and sugar cane bagasse by-products as biosorbents for the removal of cadmium from aqueous solution. Food Res Int 73:169–175
Mourabet M, El-Rhilassi A, El-Boujaady H, Bennani-Ziatni M, El-Hamri R, Taitii A (2012) Removal of fluoride from aqueous solution by adsorption on apatitic tricalcium phosphate using Box-Behnken design and desirability function. Appl Surf Sci 258:4402–4410
Muthirulan P, Kannan N, Meenakshsisundaram M (2013) Synthesis and corrosion protection properties of poly(o-phenylenediamine) nanofibers. J Adv Res 4:385–392
Nakamoto K (2009) Infrared and Raman spectra of inorganic and coordination compounds—part A: theory and applications in inorganic chemistry, 6th edn. John Wiley & Sons, Inc, Hoboken
Phuengprasop T, Sittiwong J, Unob F (2011) Removal of heavy metal ions by iron oxide coated sewage sludge. J Hazard Mater 186:502–507
Rahman N, Haseen U (2014) Equilibrium modeling, kinetic and thermodynamic studies on adsorption of Pb (II) by a hybrid inorganic-organic material :polyacrylamide zirconium (IV) iodate. Ind Eng Chem Res 53:8198–8207
Rahman N, Haseen U (2015) Development of polyacrylamide chromium oxide as a new sorbent for solid phase extraction of As (III) from food and environmental water samples. RSC Adv 5:7311–7323
Rahman N, Khan MF (2016) Nitrate removal using poly-o-toluidine zirconium (IV) ethylenediamine as adsorbent: batch and fixed-bed column adsorption modeling. J Water Process Eng 9:254–266
Rahman N, Nasir M (2017) Development of Zr(IV) – doped polypyrrole/zirconium(IV) iodate composite for efficient removal of fluoride from water environment. J Water Process Eng 19:172–184
Rahman N, Haseen U, Khan MF (2015) Cyclic tetra [(indolyl)-tetramethyl]-diethane-1,2-diamine (CTet) impregnated hydrous zirconium oxide as a novel hybrid material for enhanced removal of fluoride from water samples. RSC Adv 5:39062–39074
Rahmanidn B, Pakizeh M, Maskooki A (2012) Optimization of lead removal from aqueous solution by micellar-enhanced ultrafiltration process using Box-Behnken design. Korean J Chem Eng 29:804–811
Rao KS, Mohapatra M, Anand S, Venkateswarlu P (2010) Review on cadmium removal from aqueous solutions. Int J Eng Sci Technol 2:81–103
Rocha CG, Zaia DAM, Alfaya RVDS, Alfaya AADS (2009) Use of rice straw as biosorbent for removal of Cu(II), Zn(II), Cd(II) and Hg(II) ions in industrial effluents. J Hazard Mater 166:383–388
Rodriguez LA, Maschio LJ, da Silva RE, da Silva MLCP (2010) Adsorption of Cr(VI) from aqueous solution by hydrous zirconium oxide. J Hazard Mater 173:630–636
Sall ML, Diaw AKD, Sall DG, Biraud AC, Oturan N, Oturan MA, Fourdrin C, Huguenot D, Aason JJ (2018) Removal of lead and cadmium from aqueous solution by using 4-amino-3-hydroxynaphthalene sulfonic acid-doped polypyrrole films. Environ Sci Pollut Res 25:8581–8591
Salmani MH, Davoodi M, Ehrampoush MH, Ganeian MT, Fallahzadah MH (2013) Removal of cadmium (II) from simulated wastewater by ion floatation technique. Iranian J Environ Health Sci Eng 10:16. https://doi.org/10.1186/735-2746-10-16.
Samanta S, Roy P, Kar P (2017) Influence of structure of poly (o-phenylenediamine) on the do** ability and conducting property. Ionics 23:937–947
Seredych M, Bandosz TJ (2010) Effect of surface features on adsorption of SO2 on graphite oxide/Zr (OH)4 composites. J Phys Chem C 114:14552–14560
Singanan M (2011) Removal of lead (II) and cadmium (II) from wastewater using activated biocarbon. Sci Asia 37:115–119
Singh P, Rawat JP, Rahman N (2003) Synthesis and characterization of zirconium (IV) iodovanadate and its use as electron exchanger. Talanta 59:443–452
Singh R, Chadetrik R, Kumar R, Bishnoi K, Bhatia D, Kumar A, Bishnoi NR, Singh N (2010) Biosorption optimization of lead(II), cadmium(II) and copper(II) using response surface methodology and applicability in isotherm and thermodynamics modelling. J Hazard Mater 174:623–634
Socrates G (1980) Infrared Characteristic Group Frequencies. John Wiley & Sons, Ltd., Bristol, p 53:54,84
Soenarjo S, Wijaya C (2006) Adsorption behaviour of cadmium (II) on hydrous oxide inorganic resins. J Sains dan Teknologi Nuklir, Indonesia 7:131–145
Soltani R, Dinari M, Mohammadnezhad G (2018) Ultrasonic-assisted synthesis of novel nanocomposite of poly(vinyl alcohol) and amino-modified MCM-41: a green adsorbent for Cd(II) removal. Ultrason Sonochem 40:533–542
Tak B-Y, Tak B-S, Park Y-J, Yoon Y-H (2015) Optimization of color and COD removal from livestock wastewater by electrocogulation process: application of Box-Behnken design (BBD). J Ind Eng Chem 28:307–315
Talebi M, Abbasizadeh S, Ali R (2017) Evaluation of single and simultaneous thorium and uranium sorption from water systems by an electrospun PVA/SA/PEO/HZSM5 nanofiber. Process Saf Environ Prot 109:340–356
Tehrani MM, Abbasizadeh S, Alamdari A (2017) Prediction of simultaneous sorption of copper (II), cobalt (II) and zinc (II) contaminants from water system by a novel multi-functionalized zirconia nanofiber. Desalin Water Treat 62:340–417
US Environmental Protection Agency, International risk information system (IRIS) (1999) On cadmium. National centre for environmental assessment, Office of research and development, Washington, DC
Vekateswarlu S, Yoon M (2015) Rapid removal of cadmium ions using green-synthesized Fe3O4 nanoparticles capped with diethyl-4-(4amino-5-mercapto-4 H-1,2,4,-triazol-3-yl) phenyl phosphonate. RSC Adv 5:65444–65453
Venkatesan G, Senthilnthan U (2013) Adsorption batch studies on the removal of cadmium using wood of Derris Indica based activated carbon. Res J Chem Environ 17:19–24
Venkateswarlu S, Yoon M (2015) Rapid removal of cadmium ions using green-synthesized Fe3O4 nanoparticles capped with diethyl-4-(4amino-5-mercapto-4H-1,2,4-triazol-3yl) phenyl phosphonate. RSC Adv 5:65444–65453
Vilayatkar ND, Rahangdale PK, Donadkar DK (2016) Adsorption of cadmium (II) from solution onto activated carbon prepared from Madhucalongifolia fruit shell. Int J Adv Res 4:1360–1364
Wang C-W, Baroord JP, Mckay G (2014) Kinetic and equilibrium studies for the removal of cadmium ions by ion exchange resin. J Environ Chem Eng 2:698–707
Wang K, Zhao J, Li H, Zhang X, Shi H (2016) Removal of cadmium (II) from aqueous solution by granular activated carbon supported magnesium hydroxide. J Taiwan Inst Chem Eng 6:287–291
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div Am Soc Civ Eng 89:31–59
World Health Organization (2011) Cadmium in drinking-water, background document for development of WHO Guidelines for drinking-water quality
Wu F-C, Tseng R-L, Juang R-S (2009) Characteristics of Elovich equation used for the analysis of adsorption kinetics in dye-chitosan systems. Chem Eng J 150:366–373
Xu L, Zhuang Z (2014) Removal of cadmium ions from aqueous solution using chemically modified peanut shell. J Chem Pharm Res 6:646–653
Zhang JY, Wei Y, Li H, Zeng EY, You J (2017) Application of Box-Behnken design to optimize multi-solid phase extraction for trace neonicotinoids in water containing high level of matrix substances. Talanta 170:392–398
Zheng L, Meng P (2016) Preparation, characterization of corn stalk xanthates and its feasibility for Cd (II) removal from aqueous solution. J Taiwan Inst Chem Eng 58:391–400
Zong E, Wei D, Wan H, Zheng S, Xu Z, Zhu D (2013) Adsorption removal of phosphate ions from aqueous solution using zirconia-functionalized graphite oxide. Chem Eng J 221:193–203
Acknowledgements
The authors are grateful to UGC (DRS-II) and DST (FIST and PURSE Phase II) for providing necessary research facilities. One of the authors (Mohd Nasir) is thankful to UGC for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Tito Roberto Cadaval Jr
Electronic supplementary material
ESM 1
(DOCX 256 kb)
Rights and permissions
About this article
Cite this article
Rahman, N., Nasir, M. Application of Box–Behnken design and desirability function in the optimization of Cd(II) removal from aqueous solution using poly(o-phenylenediamine)/hydrous zirconium oxide composite: equilibrium modeling, kinetic and thermodynamic studies. Environ Sci Pollut Res 25, 26114–26134 (2018). https://doi.org/10.1007/s11356-018-2566-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2566-1