Abstract
Introduction
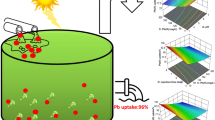
The application of response surface methodology is presented for optimizing the removal of U ions from aqueous solutions using Padina sp., a brown marine algal biomass.
Methods
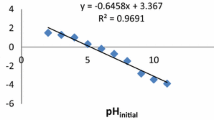
Box–Wilson central composite design was employed to assess individual and interactive effects of the four main parameters (pH and initial uranium concentration in solutions, contact time and temperature) on uranium uptake.
Results and discussion
Response surface analysis showed that the data were adequately fitted to second-order polynomial model. Analysis of variance showed a high coefficient of determination value (R 2 = 0.9746) and satisfactory second-order regression model was derived.
Conclusion
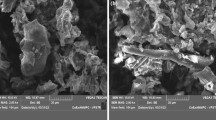
The optimum pH and initial uranium concentration in solutions, contact time and temperature were found to be 4.07, 778.48 mg/l, 74.31 min, and 37.47°C, respectively. Maximized uranium uptake was predicted and experimentally validated. The equilibrium data for biosorption of U onto the Padina sp. were well represented by the Langmuir isotherm, giving maximum monolayer adsorption capacity as high as 376.73 mg/g.






Similar content being viewed by others
Abbreviations
- b:
-
the Langmuir adsorption constant (l mg−1)
- Ceq :
-
residual metal ion concentration at equilibrium (mg l−1)
- C o :
-
initial metal ion concentration (mg l−1)
- C t :
-
metal concentration after time t (mg l−1)
- q eq :
-
adsorbed metal ion quantity per g of alga at equilibrium (mg g−1)
- q exp :
-
observed U uptake (mg/g)
- q pre :
-
predicted U uptake (mg/g)
- q t :
-
adsorbed metal ion quantity per g of alga at any time (mg g−1)
- q t,exp :
-
observed U uptake at any time (mg/g)
- q t,pre :
-
predicted U uptake at any time (mg/g)
- Q 0 :
-
the Langmuir adsorption constant (mg g−1)
- Q eq :
-
adsorbed metal ion quantity per g of alga at equilibrium (mg g−1)
- M :
-
amount of the biosorbent (g)
- R 2 :
-
correlation coefficient
- t :
-
time (min)
- T :
-
solution temperature (°C)
- v :
-
volume of the solution (l)
- w :
-
algae concentration (g l−1)
References
Bituh T, Marovic G, Franic Z, Sencar J, Bronzovic M (2009) Radioactive contamination in Croatia by phosphate fertilizer production. J Hazard Mater 162:1199–1203
Ragozzini RJ, Ross-Smith MA, Sparrow GJ, Walker GS (1986) Selective dissolution of uranium from a copper flotation concentrate. Hydrometallurgy 16:377–393
Kalin M, Wheeler WN, Meinrath G (2005) The removal of uranium from mining wastewater using algal/microbial biomass. J Environ Radioactiv 78:151
Bayramoglu G, Celik G, Yakup Arica M (2006) Studies on accumulation of uranium by fungus Lentinus sajor-caju. J Hazard Mater B136:345–353
Parab H, Joshi S, Shenoy N, Verma R, Lali A, Sudersanan M (2005) Uranium removal from aqueous solution by coir pith: Equilibrium and kinetic studies. Biores Technol 96:1241
Li P, Mao Z, Rao X, Wang X, Min M, Qiu L, Liu Z (2004) Biosorption of uranium by lake-harvested biomass from a cyanobacterium bloom. Biores Technol 94:193–195
Psareva TS, Zakutevskyy OI, Chubar NI, Strelko VV, Shaposhnikova TO, Carvalho JR, Correia MJN (2005) Uranium sorption on cork biomass. Colloids Surf A Physicochem Eng Asp 252:231–236
Sar P, Kazy SK, D’Souza SF (2004) Radionuclide remediation using a bacterial biosorbent. Int Biodeterior Biodegrad 54:193–202
Genc O, Yalcınkaya Y, Buyuktuncel E, Denizli A, Arıca MY, Bektas S (2003) Uranium recovery by immobilized and dried powdered biomass: Characterization and comparison. Int J Miner Process 68:93–107
Yang J, Volesky B (1999) Biosorption of uranium on sargassum biomass. Wat Res 33:3357–3363
Khani MH, Keshtkar AR, Meysami B, Zarea MF, Jalali R (2006) Biosorption of uranium from aqueous solutions by nonliving biomass of marinealgae Cystoseira indica. Electron J Biotechnol 9:100–106
Khani MH, Keshtkar AR, Ghannadi M, Pahlavanzadeh H (2008) Equilibrium, kinetic and thermodynamic study of the biosorption of uranium onto Cystoseria indica algae. J Hazard Mater B136:612–618
Sar P, D’Souza SF (2001) Biosorptive uranium uptake by a Pseudomona: Characterization and equilibrium studies. J Chem Technol Biotechnol 76:1286
Massart DL et al (2003) Handbook of chemometrics and qualimetrics, part A. Elsevier, Amsterdam
Ferreira SLC et al (2004) Doehlert matrix: a Chemometric tool for analytical chemistry—review. Talanta 63:1061–1067
NIST/SEMATECH e-Handbook of Statistical Methods. Available at: http://www.itl.nist.gov/div898/handbook/, created date: 6/01/2003
Tan IAW, Ahmad AL, Hameed BH (2008) Adsorption of basic dye on high-surface-area activated carbon prepared from coconut husk: Equilibrium, kinetic and thermodynamic studies. J Hazard Mater 154:337–346
Hameed BH, Tan IAW, Ahmad AL (2008) Optimization of basic dye removal by oil palm fiber-based activated carbon using response surface methodology. J Hazard Mater 153:324–332
Tarangini K, Kumar A, Satpathy GR, Sangal VK (2009) Statistical optimization of process parameters for Cr (VI) biosorption onto mixed cultures of Pseudomonas aeruginosa and Bacillus subtilis. Clean 37:319–327
Box GEP, Hunter JS (1957) Multi-factor experimental designs for exploring response surfaces. Ann Math Stat 28:195–241
Kumar A, Prasad B, Mishra IM (2007) Process parametric study for ethene carboxylic acid removal onto powder activated carbon using Box–Behnken design. Chem Eng Technol 30:932–937
Kumar M, Anto Ponselvana FI, Ram Malviyaa J, Chandra Srivastavaa V, Deo Mall I (2009) Treatment of biodigester effluent by electrocoagulation using iron electrodes. J Hazard Mater 165:345–352
Figueira MM, Volesky B, Mathieu HJ (1999) Instrumental analysis study of Iron species biosorption by Sargassum biomass. Environ Sci Technol 33:1840–1846
Silke S, Volesky B (1995) Modelling of the proton–metal ion exchange in biosorption. Environ Sci Technol 29:3049–3058
Heng TL (2004) Biosorption of chromium by locally-derived marine algal biomass, Master Engineering Thesis, National University of Singapore, pp 85
Davis TA, Volesky B, Mucci A (2003) A review of the biochemistry of heavy metal biosorption by brown algae. Water Res 37:4311–4330
Murugesan GS, Sathishirkumar M, Suaminathan K (2006) Arsenic removal from groundwater by pretreated waste tea fungal biomass. Bioresour Technol 97:483–487
Fiol N, Villascusa I, Martinez M, Mirralles N, Poch J, Seralos J (2006) Sorpton of Pb II, Ni II, Cu II and Cd II from aqueous solutions by olive stone waste. Sep Purif Technol 50:132–140
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Khani, M.H. Statistical analysis and isotherm study of uranium biosorption by Padina sp. algae biomass. Environ Sci Pollut Res 18, 790–799 (2011). https://doi.org/10.1007/s11356-010-0425-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-010-0425-9