Abstract
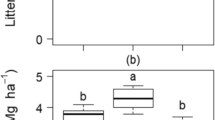
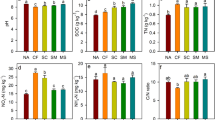
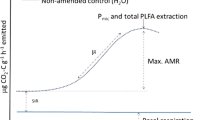
The soils of Lakshadweep Islands are formed as a result of the fragmentation of coral limestone, that is carbonate-rich, with neutral pH, but poor in plant nutrients. Coconut palm (Cocos nucifera L.) is the main crop cultivated, supporting the life and livelihood of the islanders. No external fertilizer application or major plant protection measures are adopted for their cultivation as the Islands were declared to go organic decades back. Yet, Lakshadweep has one of the highest productivity of coconut compared with other coconut growing areas in India. Therefore, a question arises: how is such a high coconut productivity sustained? We try to answer by estimating in three main islands (i) the nutrients added to the soil via the litter generated by coconut palms and (ii) the role of soil microbiota, including arbuscular mycorrhizae, for the high productivity. Our results indicated that, besides adding a substantial quantum of organic carbon, twice the needed amount of nitrogen, extra 20% phosphorus to the already P-rich soils, 43–45% of potassium required by palms could be easily met by the total coconut biomass residues returned to the soil. Principal Component Analysis showed that soil organic carbon %, potassium, and organic carbon added via the palm litter and AM spore load scored >± 0.95 in PC1, whereas, available K in the soil, bacteria, actinomycetes, phosphate solubilizers and fluorescent pseudomonads scored above >± 0.95 in PC2. Based on our analysis, we suggest that the autochthonous nutrients added via the coconut biomass residues, recycled by the soil microbial communities, could be one of the main reasons for sustaining a high productivity of the coconut palms in Lakshadweep Islands, in the absence of any external fertilizer application, mimicking a semi-closed-loop forest ecosystem.

Similar content being viewed by others
References
Ambili K, Thomas GV, Indu P, Gopal M, Gupta A (2012) Distribution of arbuscular mycorrhizae associated with coconut and arecanut based crop** systems. J Agri Res. 1(4):338–345
Anbu P, Kang CH, Shin YJ, So JH (2016) Formations of calcium carbonate minerals by bacteria and its multiple applications. Springerplus 5:250. https://doi.org/10.1186/s40064-016-1869-2
Ashby SF (1907) Some Observations on the assimilation of atmospheric nitrogen by free living soil organism Azotobacter chroococcum of Beijerinck. J Agr Sci 2:35–51
Baillie IE, Floyd CN, Hallett SH, Andrews R (2021) Topographic and polycyclic pedogenesis in the northern atolls of the Chagos Archipelago, Indian Ocean. Geoderma 26:e00391
Barnes PW, Throop HL, Archer SR, Breshears DD, McCulley RL, Tobler MA (2015) Sunlight and soil–litter mixing: drivers of litter decomposition in drylands. Prog Bot 76:273–302
Batianoff GN, Naylor GC, Fensham RJ, Neldner VJ (2010) Characteristics of coral cay soils at Coringa-Herald Coral Sea Islands. Australia. Pacific Sci 64(2):335–347. https://doi.org/10.2984/64.2.335
Beddard FE (1903) The earthworms of the Maldive and Laccadive Islands. Fauna Geol Maldive and Laccadive Archipel 1:374–375
Bhattacharyya PN, Jha DK (2012) Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J Microbiol Biotechnol 28:1327–1350. https://doi.org/10.1007/s11274-011-0979-9
Bhattacharyya T, Pal DK, Chandran P, Ray SC, Mandal C, Telpande B (2008) Soil carbon storage capacity as a tool to prioritize areas for carbon sequestration. Curr Sci 95:482–484
Biddappa CC, Upadhyay AK, Hedge MR, Palaniswami C (1996) Organic matter recycling in plantation crops. J Plantation Crops 24(2):71–85
Bopaiah MG, Cecil SR (1993) Effect of NPK fertilizers on coconut grown on coral soils of Lakshadweep. In: Nair MK (ed) Advances in Coconut Research and Development. Oxford & IBH Publishing Co Pvt, New Delhi, pp 423–426
Deenik JL, Yost RS (2006) Chemical properties of atoll soils in the Marshall Islands and constraints to crop production. Geoderma 136(3–4):666–681
Denison RF, Kiers ET (2011) Life histories of symbiotic rhizobia and mycorrhizal fungi. Curr Biol 21:R775–R785
Devakumar M, Anand KB, Poornima V, Gopal M, Gupta A (2020) Property enhancement of recycled coarse aggregate using bio-treatment approach. Materials Today: Proceedings, https://doi.org/10.1016/j.matpr.2020.10.662
Feby A, Nair S (2010) Sponge-associated bacteria of Lakshadweep coral reefs, India: resource for extracellular hydrolytic enzymes. Adv Biosci Biotechnol 1:330–337. https://doi.org/10.4236/abb.2010.14043
Fiore CL, Jarett JK, Olson ND, Lesser MP (2010) Nitrogen fixation and nitrogen transformations in marine symbioses. Trends Microbiol 18(10):455–463
Frąc M, Hannula SE, Bełka M, Jędryczka M (2018) Fungal biodiversity and their role in soil health. Front Microbiol 9:707. https://doi.org/10.3389/fmicb.2018.00707
Gallo ME, Porras-Alfaro A, Odenbach KJ, Sinsabaugh RL (2009) Photoacceleration of plant litter decomposition in an arid environment. Soil Biol Biochem 41:1433–1441
George M, Anjumol A, George G, Mohamed-Hatha AA (2012) Distribution and bioactive potential of soil actinomycetes from different ecological habitats. African J Microbiol Res. 6:2265–2271
George P, Gupta A, Gopal M, Thomas L, Thomas GV (2013) Multifarious beneficial traits and plant growth promoting potential of Serratia marcescens KiSII and Enterobacter sp. RNF 267 isolated from the rhizosphere of coconut palms (Cocos nucifera L.). World J Microbiol Biotechnol 29:109–117
George P, Gupta A, Gopal M, Thomas L, Thomas GV (2018) Systematic screening strategies for identifying elite plant growth promoting rhizobacteria for coconut (Cocos nucifera L.). Int J Curr Microbiol App Sci 7:1051–1074
Gerdemann JW, Nicholson TH (1963) Spores of mycorrhizal endospore species extracted from soil by wet sieving and decanting. Trans British Mycol Soc 46:235–244
Gopal M (2000) Glimpses of coconut cultivation in Andrott Island of Lakshadweep. Indian Coconut J 30(10):1–3
Gopal M, Gupta A, Rajan P, Radhakrishnan Nair CP (2001) Effect of systemic soil insecticides and a plant product on microbial load of soil in root (wilt) affected coconut monocrop** ecosystem. CORD 17(1):52–71
Gopi M, Ajith Kumar TT, Balagurunathan R, Vinoth R, Dhaneesh KV, Rajasekaran R, Balasubramanian T (2012) Phylogenetic study of sponge associated bacteria from the Lakshadweep archipelago and the antimicrobial activities of their secondary metabolites. World J Microbiol Biotechnol 28(2):761–766. https://doi.org/10.1007/s11274-011-0860-x
Gopinath A, Nair SM, Kumar NC et al (2010) A baseline study of trace metals in a coral reef sedimentary environment, Lakshadweep Archipelago. Environ Earth Sci 59:1245–1266. https://doi.org/10.1007/s12665-009-0113-6
Gupta A, Gopal M, Tilak KVBR (2000) Mechanism of plant growth promotion by rhizobacteria. Indian J Exptl Biol 38:856–862
Haldar KR, Dhani S, Mandal CK (2007) On some earthworms present in unnamed collections of zoological survey of India. Rec Zool Survey India 107(3):79–93
Hanway JJ, Heidel H (1952) Soil analysis method as used in lowa state college soil testing laboratory. Iowa State Coll Agric 57:1–31
Harter RD (2007) Acid soils of the tropics. ECHO Technical Note, pp 11, http://courses.umass.edu/psoil370/Syllabus-files/Acid_Soils_of_the_Tropics.pdf
Hodge A (2014) Interactions between arbuscular mycorrhizal fungi and organic material substrates. Adv Appl Microbiol 89:47–99. https://doi.org/10.1016/B978-0-12-800259-9.00002-0
IIRS (2010) Biodiversity Characterization at Landscape Level in North West India and Lakshadweep Islands Using Satellite Remote Sensing and Geographic Information System 978–81–211–0774–7, National Remote Sensing Centre, Hyderabad, India
Jackson ML (1973) Soil Chemical Analysis. Prentice Hall of India Pvt. Ltd., New Delhi
Jacob PM, Krishnamoorthy B (1981) Coconut genotypes of Lakshadweep islands. Paper presented in the 4th Plantation Crops Symposium, Mysore, India, 1981. PLACROSYM IV:3–8
James PSBR (2011) Lakshadweep : Islands of ecological fragility, environmental sensitivity and anthropogenic vulnerability. J Coastal Environ 2(1):9–25
Johnston A (1949) Vesicular arbuscular mycorrhizae in sea island cotton and other tropical plants. J Trop Agrl. 26:118–121
Joseph John K, Nair AR, Suma A, Unnikrishnan M, Arunachalam V (2018) Agro-biodiversity and ethnobotany of Lakshadweep Islands of India. Genet Resour Crop Evol 65:2083–2094. https://doi.org/10.1007/s10722-018-0676-8
Jothimani S (1994) Organic farming in coconut. Indian Coconut Journal 25(7):48–49
Joy A, Anoop P, Rajesh R, Mathew J, Mathew A, Gopinath A (2019) Spatial variation of phosphorus fractionation in the coral reef sediments of Lakshadweep Archipelago, Indian. Ocean Chem Ecol 35(7):592–612
Kaladharan P (2001) Seaweed resource potential of Lakshadweep. Geol S India Spec Publ 56:121–124
Kasana RC, Salwan R, Dhar H, Dutt S, Gulati A (2008) A rapid and easy method for the detection of microbial cellulases on agar plates using gram’s iodine. Curr Microbiol 57(5):503–507. https://doi.org/10.1007/s00284-008-9276-8
Krishnan P, Nair KM, Naidu LGK, Srinivas S, Koyal A, Nasre RA, Ramesh M, Gajbhiye KS (2004) Land, soil and land use of Lakshadweep Coral Islands. J Indian Soc Soil Sci 52(3):226–231
Li S-P, Wang P, Chen Y et al (2020) Island biogeography of soil bacteria and fungi: similar patterns, but different mechanisms. ISME J 14:1886–1896
Lilly VG (1975) Note on the development of vesicular arbuscular mycorrhiza Endogone fasciculata in coconut roots. Curr Sci 44:201–202
Lindsay WL, Norvell WA (1978) Development of a DTPA soil test for zinc, iron, manganese and copper. Soil Sci Soc Amer J. 42:421–428
Lloyd H, Zar KH, Karr JR (1968) On the calculation of information- theoretical measures of diversity [J]. Am mid Nat 79:257–272
Ma Z, Yang W, Wu F, Tan B (2017) Effects of light intensity on litter decomposition in a subtropical region. Ecosphere 8(4):e01770. https://doi.org/10.1002/ecs2.1770
Mandal D, Tripathi KP (2009) Soil erosion limits of Lakshadweep Archipelago. Curr Sci 96(2):276–280
Manciot E, Ollagnier M, and Ochs R (1979) Mineral nutrition of the coconut around the world. Oléagineux. 34:511–515, 576–580
Malhotra SK, Maheshwarappa HP, Selvamani V, Chowdappa P (2017) Diagnosis and management of soil fertility constraints in coconut (Cocos nucifera): a review. Indian J Agrl Sci. 87(6):711–726
Mallela J, Lewis SE, Croke B (2013) Coral skeletons provide historical evidence of phosphorus runoff on the Great Barrier Reef. PLoS ONE. https://doi.org/10.1371/journal.pone.0075663
McGee PA (1989) Vesicular-arbuscular mycorrhizal and saprophytic fungi of the Swain Reefs. Australia Mycological Res 93(3):375–378
Mendes R, Kruijt K, deBruijn I, Dekkers E, Van Der Voort M, Schneider JHM et al (2011) Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 332:1097–1100. https://doi.org/10.1126/science.1203980
Menon RS, Mohan S (2016) Diversity of Soil Microbes in Amini Island of Lakshadweep, Proceedings of the 25th Swadeshi Science Congress [ISBN: 978–81- 928129–3–9] pp: 382–386.
Mitra A, Sundaresan J, Syed Ali K, Pal N, Datta U, Mitra A, Paramanick P, Zaman S (2017) Baseline data of stored carbon in Spinifex littoreus from Kadmath Island, Lakshadweep. In: Goel M, Sudhakar M (eds) Carbon Utilization Green Energy and Technology. Springer, Singapore
Nair KM, Haris A, Mathew J, Srinivasan V, Dinesh R, Hamza H, Subramanian P, Thamban C, Chandran KP, Krishnakumar V, Bhat R, Hegde R, Singh SK (2018) Coconut-growing soils of Kerala: 2. Assessment of fertility and soil related constraints to coconut production. J Plant Crops 46(2):84–91
Nasmia RE, Masyahoro A, Putera FHA, Natsir S (2021) The utilization of seaweed-based liquid organic fertilizer to stimulate Gracilaria verrucosa growth and quality. Int J Environ Sci Technol 18:1637–1644. https://doi.org/10.1007/s13762-020-02921-8
Nelliat EV (1973) NPK nutrition of coconut palm. A review. J Plantation Crops, Suppl. 1:70–80
Nelson L-A, Cade-Menun BJ, Walker IJ, Sanborn P (2020) Soil phosphorus dynamics across a holocene chronosequence of aeolian sand dunes in a hypermaritime environment on Calvert Island, BC, Canada. Front for Glob Change 3:83. https://doi.org/10.3389/ffgc.2020.00083
Olsen S, Cole C, Watanabe F, Dean L (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. USDA Circular Nr 939, US Gov. Print. Office, Washington, D.C.
Ong CS, Juan JC, Yule CM (2017) The contribution of leaching to nutrient release from leaf litter of two emergent tree species in a Malaysian tropical peat swamp forest. Hydrobiologia 794(1):125–137. https://doi.org/10.1007/s10750-017-3086-6
Ouvrier M, de Taffin G (1985) Evolution of mineral elements of coconut husks left in the field. Oléagineux 40(8 & 9):423–430
Pande S, Sant NR, Ranade SD, Pednekar SN, Mestry PG, Kharat SS, Deshmukh V (2007) An ornithological expedition to the Lakshadweep archipelago: Assessment of threats to pelagic and other birds and recommendations. Indian Birds 3(1):2–12
Pandey S, Gupta S (2020) Diversity analysis of ACC deaminase producing bacteria associated with rhizosphere of coconut tree (Cocos nucifera L.) grown in Lakshadweep islands of India and their ability to promote plant growth under saline conditions. J Biotechnol 324:183–197
Patil JL, Haldanka PM, Jamadagni BM, Salv MJ (1993) Variability and correlation studies for nut characters in coconut. J Mahrashtra Agrl University 18(3):361–364
Philips JM, Hayman DS (1970) Improved procedures for cleaning roots and staining parasitic and vesicular-arbuscular fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158–161
Rajagopal V, Kasturi Bai KV, Voleti SR (1990) Screening of coconut genotype for drought tolerance. Oléagineux 45:215–223
Rajeshkumar PP, Thomas GV, Gupta A, Gopal M (2015) Diversity, richness and degree of colonization of arbuscular mycorrhizal fungi in coconut cultivated along with intercrops in high productive zone of Kerala, India. Symbiosis 65:125–141
Ramírez-Silva JH, Cortazar-Ríos M, Ramírez-Jaramillo G, Oropeza-Salín CM, Rondón-Rivera DD (2021) Soil organic matter and nitrogen content as related to coconut nutrition in Guerrero. Mexico Open Access Libr J 8:e7727. https://doi.org/10.4236/oalib.1107727
Ratnambal MJ, Nair MK, Muralidharan K, Kumaran PM, Rao EVVB, Pillai RV (1995) Coconut Descriptors Part-I. CPCRI, Kasaragod, pp 168–175
Ricci F, Marcelino VR, Blackall LL, Kuhl M, Medina M, Verbruggen H (2019) Beneath the surface: community assembly and functions of the coral skeleton microbiome. Microbiome 7:159. https://doi.org/10.1186/s40168-019-0762-y
Robertson GP, Paul EA (1999) Decomposition and soil organic matter dynamics. In: Sala OE, Jackson RB, Mooney HA, Howarth RW (eds) Methods of ecosystem science. Springer, New York, pp 104–116
Ruiz-Lozano JM, Porcel R, Aroca R (2006) Does the enhanced tolerance of arbuscular mycorrhizal plants to water deficit involve modulation of drought-induced plant genes? New Phytol 171(4):693–698. https://doi.org/10.1111/j.1469-8137.2006.01841.x
Selvamani V, Duraisami V (2014) Identifying and map** soil fertility constraints for coconut in Coimbatore and Tiruppur districts of Tamil Nadu state. Indian J Plantation Crops 42(3):348–357
Shalev O, Karasov TL, Lundberg DS et al (2022) Commensal Pseudomonas strains facilitate protective response against pathogens in the host plant. Nat Ecol Evol. https://doi.org/10.1038/s41559-022-01673-7
Shameena Beegum PP, Thamban C, Subramanian P, Mathew AC, Ananth PN (2022) Coconut cultivation and coconut based enterprises in Lakshadweep - Changing scenario and need for revitalizing coconut sector. Indian Coconut J 64(9):5–12
Shutenko GS Andriuzzi WS, Dyckmans J, Luo Y, Wilkinson TL, Schmidt O (2022) Rapid transfer of C and N excreted by decomposer soil animals to plants and above-ground herbivores. Soil Biol Biochem. https://doi.org/10.1016/j.soilbio.2022.108582
Simpson EH (1949) Measurement of diversity. Nature 163:688
Singh KD, Velayutham M (1979) Nutrient indexing in coconut leaves and soils of Lakshadweep islands. 1. The need for potash and nitrogen. Indian Coconut J 10(8):1–3
Smith V (2008) Energy flow and nutrient cycling in the Marion Island terrestrial ecosystem: 30 years on. Polar Rec 44(3):211–226. https://doi.org/10.1017/S0032247407007218
Soni VK (2021) Long term variation in chemical composition of precipitation and wet deposition of major ions at Minicoy and Port Blair : Islands in Arabian Sea and Bay of Bengal. Mausam 57(3):489–498
Sullivan DM, Moore AD, Verhoeven E, Brewer LJ (2020) Baseline soil nitrogen mineralization: Measurement and interpretation (EM 9281). Oregon State University Extension, Corvallis, OR
Szmant-Froelich A (1983) Functional aspects of nutrient cycling on coral reefs. NOAA Symp Ser Undersea Res. 1:133–139
Takahashi Y, Omura S (2003) Isolation of new actinomycete strains for the screening of new bioactive compounds. J Gen Appl Microbiol 49(3):141–154
Thamban C, Samsudeen K, Shameena Beegum PP (2020) Coconut farming in Lakshadweep Islands - strategies for enhancing sustainability. Technical Bulletin No. 149. ICAR-Central Plantation Crops Research Institute, Kasaragod. p 28
Thomas GV, Ghai SK (1987) Genotype dependent variation in vesicular-arbuscular mycorrhizal colonization of coconut seedlings. Proc Indian Acad Sci (Pl Sci) 4:289–294
Tyagi AP (2009) Solar radiant energy over India. India Meteorological Department, Ministry of Earth Sciences, India, p 4179
Vadivelu S (1979) A hypothesis on the formation of Lakshadweep Islands from pedogenic standpoint. Agropedia 8:1–9
Vadivelu S, Bandyopadhyay AK (1997) Characteristics, genesis and classification, of soils of Minicoy Island. Lakshadweep Indian J Soil Sci 45(6):796–801
Walkley AJ, Black IA (1934) Estimation of soil organic carbon by the chromic acid titration method. Soil Sci 37:29–38
Young HS, McCauley DJ, Dunbar RB, Dirzo R (2009) Plants cause ecosystem nutrient depletion via the interruption of bird-derived spatial subsidies. PNAS 107(5):2072–2077. https://www.pnas.org/cgi/content/full/0914169107/DCSupplemental
Yousuf J, Thajudeen J, Rahiman M, Krishnankutty S, Alikunj AP, Abdulla MHA (2017) Nitrogen fixing potential of various heterotrophic Bacillus strains from a tropical estuary and adjacent coastal regions. J Basic Microbiol 57(11):922–932. https://doi.org/10.1002/jobm.201700072
Wilkes TI (2021) Arbuscular mycorrhizal fungi in agriculture. Encyclopedia 1(4):1132–1154. https://doi.org/10.3390/encyclopedia1040085
Wilkes TI, Warner DJ, Edmonds-Brown V, Davies KG, Denholm I (2021) Zero tillage systems conserve arbuscular mycorrhizal fungi, enhancing soil glomalin and water stable aggregates with implications for soil stability. Soil Syst 5:4
Wolanski E (2009) Oceans and Aquatic ecosystems Vol II, Encyclopedia of Life Support System. An UNESCO publication. pp 358
Zheng BX, Zhang DP, Wang Y et al (2019) Responses to soil pH gradients of inorganic phosphate solubilizing bacteria community. Sci Rep 9:25. https://doi.org/10.1038/s41598-018-37003-w
Zheng Y, Maitra P, Gan H-Y, Chen L, Li S, Tu T, Chen L, Mi X, Gao C, Zhang G-D (2021) Soil fungal diversity and community assembly: affected by island size or type? FEMS Microbiol Ecology. https://doi.org/10.1093/femsec/fiab062
Acknowledgements
The authors sincerely thank the Indian Council of Agricultural Research (ICAR), New Delhi, for funding Network Project on “Application of Microorganisms in Agriculture and Allied Sector (AMAAS)” under which this study was undertaken. Thanks are due to Mr. M.I. Arif, Senior Technician, ICAR-Central Plantation Crops Research Institute, Minicoy (presently with ICAR-Central Island Agricultural Research Institute), for hel** in the collection of soil samples at Minicoy, Kalpeni and Kavaratti. The authors are grateful to the anonymous reviewers for their critical comments which helped to improve the ms significantly.
Author information
Authors and Affiliations
Contributions
MG and AG had conceptualized the hypothesis and the work plan. MG had collected soil samples with the support of technical staff. Vegetation diversity survey was carried out by VA and PMJ. Microbial analysis was carried out by MG and AG, soil physico-chemical properties by HPM, and statistical analysis by VA. Manuscript was written by MG, AG and VA with inputs from all authors. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gopal, M., Gupta, A., Arunachalam, V. et al. Autochthonous nutrient recycling driven by soil microbiota could be sustaining high coconut productivity in Lakshadweep Islands sans external fertilizer application. World J Microbiol Biotechnol 38, 213 (2022). https://doi.org/10.1007/s11274-022-03373-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-022-03373-7