Abstract
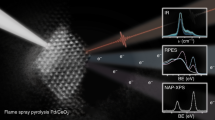
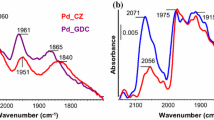
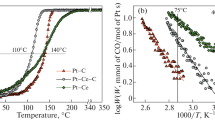
The plasma-arc (PA) method was applied for the highly efficient synthesis of Pd/Ce–Sn–O catalysts for CO oxidation. Using the PA sputtering of a graphite electrode together with Pd, Ce and Sn metallic components in inert atmosphere, a PdCeSnC composite was obtained. After the subsequent calcination in oxygen over the temperature range of 600–1000 °C, the initial composites were transformed into active catalysts of CO oxidation at low temperatures (LTO CO). Catalytic testing showed that these PA-prepared Pd/Ce–Sn–O catalysts were characterized by unusually high thermal stability. The catalysts demonstrated the excellent LTO CO performance after calcination at 1000 °C. According to the XRD and HRTEM observations, the Pd/Ce–Sn–O catalysts can be described as heterogeneous structures consisting of small CeO2 and SnO2 particles that interact with each other, forming extended grain boundaries and a composite structure. The TPR-CO and XPS methods detected highly dispersed Pd species in the active catalysts, namely Pd2+ in the lattice of ceria (a Pd-ceria solid solution) and the PdOx clusters on the surface. Deactivation of the Pd/Ce–Sn–O is governed by decomposition of the Pd-ceria solid solution accompanied by the sintering of the PdOx clusters and formation of the metallic and oxide palladium nanoparticles. Oxygen species with high mobility in the Pd/Ce–Sn–O catalyst were detected by a TPR-CO method. The amount of the highly mobile oxygen species is in five times higher for the Pd/Ce–Sn–O catalyst then for the Pd/CeO2 sample. Promising perspectives of the plasma-arc application for catalyst the synthesis of with improved properties are discussed.











Similar content being viewed by others
References
Kašpar J, Fornasiero P, Hickey N (2003) Automotive catalytic converters: current status and some perspectives. Catal Today 77:419–449
Chen H-Y, Chang H-LR (2015) Development of low temperature three-way catalysts for future fuel efficient vehicles. Johnson Matthey Technol Rev 59:64–67
Shelef M, McCabe RW (2000) Twenty-five years after introduction of automotive catalysts: what next? Catal Today 62:35–50
Zhang Z, Fan Y, **n Y et al (2015) Improvement of air/fuel ratio operating window and hydrothermal stability for Pd-only three-way catalysts through a Pd–Ce2Zr2O8 superstructure interaction. Environ Sci Technol 49:7989–7995
Trovarelli A (1996) Catalytic properties of ceria and CeO2-containing materials. Catal Rev 38:439–520
Ren Y, Shen Q, Guo Y et al (2015) Realization approach of Pd-only three-way catalysts with high catalytic performance and thermal stability. Sci China Chem 58:123–130
Gulyaev RV, Slavinskaya EM, Novopashin SA et al (2014) Highly active PdCeOx composite catalysts for low-temperature CO oxidation, prepared by plasma-arc synthesis. Appl Catal B Environ 147:132–143
Priolkar KR, Bera P, Sarode PR et al (2002) Formation of Ce1–xPdxO2–δ solid solution in combustion-synthesized Pd/CeO2 Catalyst: XRD, XPS, and EXAFS investigation. Chem Mater 14:2120–2128
Slavinskaya EM, Kardash TY, Stonkus OA, et al (2016) Metal–support interaction in Pd /CeO2 model catalysts for CO oxidation: from pulsed laser-ablated nanoparticles to highly active state of the catalyst. Catal Sci Technol 6:6650–6666
Kurnatowska M, Kepinski L, Mista W (2012) Structure evolution of nanocrystalline Ce1–xPdxO2–y mixed oxide in oxidizing and reducing atmosphere: reduction-induced activity in low-temperature CO oxidation. Appl Catal B Environ 117–118:135–147
Hinokuma S, Fujii H, Okamoto M et al (2010) Metallic Pd nanoparticles formed by Pd–O–Ce interaction: a reason for sintering-induced activation for CO oxidation. Chem Mater 22:6183–6190
Golunski S, Rajaram R, Hodge N, et al (2002) Low-temperature redox activity in co-precipitated catalysts: a comparison between gold and platinum-group metals. Catal Today pp 107–113
Cargnello M, Fornasiero P, Gorte RJ (2012) Opportunities for tailoring catalytic properties through metal-support interactions. Catal Lett 142:1043–1048
Haneda M, Mizushima T, Kakuta N (1998) Synergistic effect between Pd and nonstoichiometric cerium oxide for oxygen activation in methane oxidation. J Phys Chem B 102:6579–6587
Colussi S, Gayen A, Camellone MF, et al (2009) Nanofaceted Pd–O sites in Pd–Ce surface superstructures: enhanced activity in catalytic combustion of methane. Angew Chemie 48:8481–8484
Slavinskaya EM, Gulyaev RV, Zadesenets AV et al (2015) Low-temperature CO oxidation by Pd/CeO2 catalysts synthesized using the coprecipitation method. Appl Catal B Environ 166–167:91–103
Gulyaev RV, Kardash TY, Malykhin SE et al (2014) The local structure of PdxCe1–xO2–x–δ solid solutions. Phys Chem Chem Phys 16:13523–13539
Hegde MS, Madras G, Patil KC (2009) Noble metal ionic catalysts. Acc Chem Res 42:704–712
Di Monte R, Fornasiero P, Kašpar J, et al (2000) Stabilisation of nanostructured Ce0.2Zr0.8O2 solid solution by impregnation on Al2O3: a suitable method for the production of thermally stable oxygen storage/release promoters for three-way catalysts. Chem Commun 21:2167–2168
Reddy BM, Bharali P, Saikia P, et al (2007) Hafnium doped ceria nanocomposite oxide as a novel redox additive for three-way catalysts. J Phys Chem C Lett 1878–1881
Gupta A, Kumar A, Hegde MS, Waghmare UV (2010) Structure of Ce1–xSnxO2 and its relation to oxygen storage property from first-principles analysis. J Chem Phys 132:194702
Gupta A, Hegde MS, Priolkar KR et al (2009) Structural investigation of activated lattice oxygen in Ce1–xSnxO2 and Ce1–x–ySnxPdyO2–δ by EXAFS and DFT calculation. Chem Mater 21:5836–5847
Cargnello M, Fornasiero P, Gorte RJ (2013) Playing with structures at the nanoscale: designing catalysts by manipulation of clusters and nanocrystals as building blocks. ChemPhysChem 14:3869–3877
Masias KLS, Peck TC, Fanson PT (2015) Thermally robust core–shell material for automotive 3-way catalysis having oxygen storage capacity. RSC Adv 5:48851–48855
Onn TM, Zhang S, Arroyo-Ramirez L, et al (2015) Improved thermal stability and methane-oxidation activity of Pd/Al2O3 catalysts by atomic layer deposition of ZrO2. ACS Catal 5:5696–5701
Onn TM, Arroyo-Ramirez L, Monai M et al (2015) Modification of Pd/CeO2 catalyst by atomic layer deposition of ZrO2. Appl Catal B Environ 197:1–6
Oneill BJ, Jackson DHK, Lee J, et al (2015) Catalyst design with atomic layer deposition. ACS Catal 5:1804–1825
Junling L, Fu B, Kung MC, et al (2012) Coking- and sintering-resistant palladium catalysts achieved through atomic layer deposition. Sience 335:1205–1208
Cargnello M, Wieder NL, Montini T et al (2010) Synthesis of dispersible Pd@CeO2 core-shell nanostructures by self-assembly. J Am Chem Soc 132:1402–1409
Hinokuma S, Yamashita N, Katsuhara Y, et al (2015) CO oxidation activity of thermally stable Fe–Cu/CeO2 catalysts prepared by dual-mode arc-plasma process. Catal Sci Technol 5:3945–3952
Mihaiu S, Braileanu A, Bán M et al (2006) Sn–Ce–O advanced materials obtained by thermal decomposition of some precursors. J Optoelectron Adv Mater 8:572–575
Nguyen TB, Deloume JP, Perrichon V (2003) Study of the redox behaviour of high surface area CeO2–SnO2 solid solutions. Appl Catal A 249:273–284
Leite ER, Maciel AP, Weber IT et al (2002) Development of metal oxide nanoparticles with high stability against particle growth using a metastable solid solution. Adv Mater 14:905–908
Tolla B, Demourgues A, Isnard O et al (1999) Structural investigation of oxygen insertion within the Ce2Sn2O7–Ce2Sn2O8 pyrochlore solid solution by means of in situ neutron diffraction experiments. J Mater Chem 9:3131–3136
Playford HY, Modeshia DR, Barney ER et al (2011) Structural characterization and redox catalytic properties of cerium(IV) pyrochlore oxides. Chem Mater 23:5464–5473
Lowell S, Shields JE, Thomas MA, Thommes M (2006) Characterization of porous solids and powders: surface area, pore size and density. Springer, Netherlands
Gulyaev RV, Stadnichenko AI, Slavinskaya EM et al (2012) In situ preparation and investigation of Pd/CeO2 catalysts for the low-temperature oxidation of CO. Appl Catal A Gen 439–440:41–50
Ivanova AS, Slavinskaya EM, Gulyaev RV et al (2010) Metal–support interactions in Pt/Al2O3 and Pd/Al2O3 catalysts for CO oxidation. Appl Catal B Environ 97:57–71
Vasilchenko DB, Gulyaev RV, Slavinskaya EM et al (2016) Effect of Pd deposition procedure on activity of Pd/Ce0.5Sn0.5O2 catalysts for low-temperature CO oxidation. Catal Commun 73:34–38
Boronin AI, Slavinskaya EM, Danilova IG, et al (2009) Investigation of palladium interaction with cerium oxide and its state in catalysts for low-temperature CO oxidation. Catal Today 144:201–211
Slavinskaya EM, Stonkus OA, Gulyaev RV et al (2011) Structural and chemical states of palladium in Pd/Al2O3 catalysts under self-sustained oscillations in reaction of CO oxidation. Appl Catal A Gen 401:83–97
Acknowledgements
This work was partially supported by the Skolkovo Foundation (Grant agreement for Russian educational organizations No. 3 of December 25, 2014), RFBR Grant 14-03-01088 and budget project No. 0303-2016-0003 for Boreskov Institute of Catalysis.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kardash, T.Y., Slavinskaya, E.M., Gulyaev, R.V. et al. Enhanced Thermal Stability of Pd/Ce–Sn–O Catalysts for CO Oxidation Prepared by Plasma-Arc Synthesis. Top Catal 60, 898–913 (2017). https://doi.org/10.1007/s11244-017-0755-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-017-0755-7