Abstract
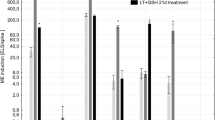
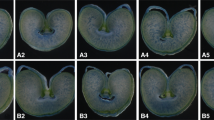
Functional damage of mitochondria and chloroplasts under stress contributes to reactive oxygen species (ROS) induced cell death. A high percentage of cell mortality during microspore culture is a limiting factor in haploid and doubled haploid plant production. In the present study, we studied the contribution of dimethyl tyrosine (DMT) conjugated short peptides to microspore embryogenesis. These DMT-peptides, which are known to translocate to subcellular targets and scavenge ROS, mitigate the effect oxidative stress plays on microspore viability and embryogenesis. The number of viable microspores was significantly higher in the presence of SS-31 and caspase-3-inhibitor (Ac-DEVD-CHO). In particular, the total number of green plant regeneration was increased by 42 % in the presence of SS-02, and by 55 % in the presence of SS-31, in triticale. Conversely, lower caspase-3-like activities were observed in the presence of SS-31 and Ac-DEVD-CHO, and intracellular ROS was reduced in the presence of SS-31, supporting the involvement of SS-31 in reducing microspore cell death by mitigating ROS and caspase-3-like activity. This study further supports the concept that antioxidant conjugated peptides offer a useful strategy for reducing ROS in plant cells.



Similar content being viewed by others
References
Alscher RG (1989) Biosynthesis and antioxidant function of glutathione in plants. Physiol Plant 77:457–464
Asif M, Eudes F, Goyal A, Amundsen EJ, Randhawa HS, Spancer DM (2013) Organelle antioxidants improve microspore embryogenesis in wheat and triticale. In Vitro Cell Dev Biol Plant 49:489–497
Baxter A, Mittler R, Suzuki N (2014) ROS as key players in plant stress signalling. J Exp Bot 65(5):1229–1240. doi:10.1093/jxb/ert375
Eudes F, Amundsen E (2005) Isolated microspore culture of Canadian 6x triticale cultivars. Plant Cell Tissue Organ Cult 82:233–241
Fortes AM, Costa J, Santos F, Segui-Simarro JM, Palme K, Altabella T, Tiburcio AF, Pais MS (2011) Arginine decarboxylase expression, polyamines biosynthesis and reactive oxygen species during organogenic nodule formation in hop. Plant signal behav 6(2):258–269
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Goyal RK, Hancock RE, Mattoo AK, Misra S (2013) Expression of an engineered heterologous antimicrobial peptide in potato alters plant development and mitigates normal abiotic and biotic responses. PLoS One 8(10):e77505. doi:10.1371/journal.pone.0077505
Halasi M, Gartel AL (2013) FOX(M1) news–it is cancer. Mol Cancer Ther 12(3):245–254. doi:10.1158/1535-7163.mct-12-0712
Hansen G (2000) Evidence for agrobacterium-induced apoptosis in maize cells. Mol Plant Microbe Interact 13(6):649–657. doi:10.1094/mpmi.2000.13.6.649
Hazell AS, Wang C (2005) Downregulation of complexin I and complexin II in the medial thalamus is blocked by N-acetylcysteine in experimental Wernicke’s encephalopathy. J Neurosci Res 79(1–2):200–207. doi:10.1002/jnr.20278
Herouart D, Van Montagu M, Inze D (1994) Developmental and environmental regulation of the Nicotiana plumbaginifolia cytosolic Cu/Zn-superoxide dismutase promoter in transgenic tobacco. Plant Physiol 104(3):873–880
Imlay JA (2008) Cellular defenses against superoxide and hydrogen peroxide. Annu Rev Biochem 77:755–776. doi:10.1146/annurev.biochem.77.061606.161055
Jacquard C, Mazeyrat-Gourbeyre F, Devaux P, Boutilier K, Baillieul F, Clement C (2009) Microspore embryogenesis in barley: anther pre-treatment stimulates plant defence gene expression. Planta 229(2):393–402. doi:10.1007/s00425-008-0838-6
Jauslin ML, Meier T, Smith RA, Murphy MP (2003) Mitochondria-targeted antioxidants protect Friedreich ataxia fibroblasts from endogenous oxidative stress more effectively than untargeted antioxidants. Faseb J 17(13):1972–1974. doi:10.1096/fj.03-0240fje
Kreslavski VD, Los DA, Allakhverdiev SI, Kuznetsov VV (2012) Signaling role of reactive oxygen species in plants under stress. Russ J Plant Physiol 59(2):141–154. doi:10.1134/S1021443712020057
Krieger-Liszkay A, Trebst A (2006) Tocopherol is the scavenger of singlet oxygen produced by the triplet states of chlorophyll in the PSII reaction centre. J Exp Bot 57(8):1677–1684. doi:10.1093/jxb/erl002
Kumlehn J, Serazetdinova L, Hensel G, Becker D, Loerz H (2006) Genetic transformation of barley (Hordeum vulgare L.) via infection of androgenetic pollen cultures with Agrobacterium tumefaciens. Plant Biotechnol J 4(2):251–261. doi:10.1111/j.1467-7652.2005.00178.x
Lam E, del Pozo O (2000) Caspase-like protease involvement in the control of plant cell death. Plant Mol Biol 44(3):417–428
Mahmudi-Azer S, Lacy P, Bablitz B, Moqbel R (1998) Inhibition of nonspecific binding of fluorescent-labelled antibodies to human eosinophils. J Immunol Methods 217(1–2):113–119
Maraschin SF, Gaussand G, Pulido A, Olmedilla A, Lamers GE, Korthout H, Spaink HP, Wang M (2005) Programmed cell death during the transition from multicellular structures to globular embryos in barley androgenesis. Planta 221(4):459–470. doi:10.1007/s00425-004-1460-x
Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9(10):490–498. doi:10.1016/j.tplants.2004.08.009
Murphy MP (1997) Selective targeting of bioactive compounds to mitochondria. Trends Biotechnol 15(8):326–330
Oleszczuk S, Sowa S, Zimny J (2004) Direct embryogenesis and green plant regeneration from isolated microspores of hexaploid triticale (x Triticosecale Wittmack) cv. Bogo. Plant Cell Rep 22(12):885–893. doi:10.1007/s00299-004-0796-9
Poljsak B (2011) Strategies for reducing or preventing the generation of oxidative stress. Oxidative Med Cell Longev 2011:194586. doi:10.1155/2011/194586
Qanungo S, Wang M, Nieminen AL (2004) N-Acetyl-l-cysteine enhances apoptosis through inhibition of nuclear factor-kappaB in hypoxic murine embryonic fibroblasts. J Biol Chem 279(48):50455–50464. doi:10.1074/jbc.M406749200
Reape TJ, McCabe PF (2010) Apoptotic-like regulation of programmed cell death in plants. Apoptosis 15(3):249–256. doi:10.1007/s10495-009-0447-2
Reddy TP, Manczak M, Calkins MJ, Mao P, Reddy AP, Shirendeb U, Park B, Reddy PH (2011) Toxicity of neurons treated with herbicides and neuroprotection by mitochondria-targeted antioxidant SS31. Int J Environ Res Public Health 8(1):203–221. doi:10.3390/ijerph8010203
Rodriguez-Serrano M, Barany I, Prem D, Coronado MJ, Risueno MC, Testillano PS (2012) NO, ROS, and cell death associated with caspase-like activity increase in stress-induced microspore embryogenesis of barley. J Exp Bot 63(5):2007–2024. doi:10.1093/jxb/err400
Roldan-Arjona T, Ariza RR (2009) Repair and tolerance of oxidative DNA damage in plants. Mutat Res 681(2–3):169–179. doi:10.1016/j.mrrev.2008.07.003
Shariatpanahi ME, Bala U, Heberle-Bors E, Touraev A (2006) Stresses applied for the re-programming of plant microspores towards in vitro embryogenesis. Physiol Plant 127:519–534
Sinha RK, Komenda J, Knoppova J, Sedlarova M, Pospisil P (2012) Small CAB-like proteins prevent formation of singlet oxygen in the damaged photosystem II complex of the cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Environ 35(4):806–818. doi:10.1111/j.1365-3040.2011.02454
Subrahmanyam NC, Kasha KJ (1975) Chromosome doubling of barley haploids by nitrous oxide and colchicine treatments. Can J Genet Cytol 17:573–578
Szeto HH (2006) Cell-permeable, mitochondrial-targeted, peptide antioxidants. AAPS J 8(2):E277–E283. doi:10.1208/aapsj080232
Touraev A, Indrianto A, Wratschko I, Vicente O, Heberle-Bors E (1996) Efficient microspore embryogenesis in wheat: Triticum aestivum L induced by starvation at high temperature. Sex Plant Reprod 9:209–215
Trabulo S, Cardoso AL, Mano M, Pedroso dL MC (2010) Cell-penetrating peptides mechanisms of cellular uptake and generation of delivery systems. Pharmaceuticals 3:961–993
Uren AG, O’Rourke K, Aravind LA, Pisabarro MT, Seshagiri S, Koonin EV, Dixit VM (2000) Identification of paracaspases and metacaspases: two ancient families of caspase-like proteins, one of which plays a key role in MALT lymphoma. Mol Cell 6(4):961–967
Vacca RA, Valenti D, Bobba A, Merafina RS, Passarella S, Marra E (2006) Cytochrome c is released in a reactive oxygen species-dependent manner and is degraded via caspase-like proteases in tobacco Bright-Yellow 2 cells en route to heat shock-induced cell death. Plant Physiol 141(1):208–219. doi:10.1104/pp.106.078683
Van Breusegem F, Dat JF (2006) Reactive oxygen species in plant cell death. Plant Physiol 141(2):384–390. doi:10.1104/pp.106.078295
Wright SN, Wang SY, Kallen RG, Wang GK (1997) Differences in steady-state inactivation between Na channel isoforms affect local anesthetic binding affinity. Biophys J 73(2):779–788. doi:10.1016/s0006-3495(97)78110-4
Wrobel J, Barlow PW, Gorka K, Nabialkowska D, Kurczynska EU (2011) Histology and symplasmic tracer distribution during development of barley androgenic embryos. Planta 233(5):873–881. doi:10.1007/s00425-010-1345-0
Wurschum T, Tucker MR, Reif JC, Maurer HP (2012) Improved efficiency of doubled haploid generation in hexaploid triticale by in vitro chromosome doubling. BMC Plant Biol 12:109. doi:10.1186/1471-2229-12-109
Xu Q, Zhang L (2009) Plant caspase-like proteases in plant programmed cell death. Plant signal behav 4(9):902–904
Yadav DK, Kruk J, Sinha RK, Pospisil P (2010) Singlet oxygen scavenging activity of plastoquinol in photosystem II of higher plants: electron paramagnetic resonance spin-trap** study. Biochim Biophys Acta 1797(11):1807–1811. doi:10.1016/j.bbabio.2010.07.003
Zadok JC, Chang TT, Konzak FC (1974) A decimal code for growth stages of cereals. Weed Res 14:415–421
Zhao K, Luo G, Zhao GM, Schiller PW, Szeto HH (2003) Transcellular transport of a highly polar 3+ net charge opioid tetrapeptide. J Pharmacol Exp Ther 304(1):425–432. doi:10.1124/jpet.102.040147
Zhao K, Zhao GM, Wu D, Soong Y, Birk AV, Schiller PW, Szeto HH (2004) Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. J Biol Chem 279(33):34682–34690. doi:10.1074/jbc.M402999200
Zhao J, Moore AN, Clifton GL, Dash PK (2005) Sulforaphane enhances aquaporin-4 expression and decreases cerebral edema following traumatic brain injury. J Neurosci Res 82(4):499–506. doi:10.1002/jnr.20649
Żur I, Dubas E, Krzewska M, Waligorski P, Dziurka M, Janowiak F (2015) Hormonal requirements for effective induction of microspore embryogenesis in triticale (3 Triticosecale Wittm.) anther cultures. Plant Cell Rep 34:47–62
Żur I, Dubas E, Krzewska M, Janowiak F, Hura K, Pociecha E, Bączek-Kwinta R, Płażek A (2014) Antioxidant activity and ROS tolerance in triticale (× Triticosecale Wittm.) anthers affect the efficiency of microspore embryogenesis. Plant Cell Tissue Organ Cult. doi:10.1007/s11240-014-0515-3
Acknowledgments
We thank Grant Duke for excellent technical assistance for confocal fluorescent microscopic studies and Eric Amundsen for excellent technical assistance for IMC and flow cytometer studies. The research reported in this paper was supported by Western Grains Research Foundation. We thank Ravinder K Goyal and Jordan Pepper for providing an English proof read.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sinha, R.K., Eudes, F. Dimethyl tyrosine conjugated peptide prevents oxidative damage and death of triticale and wheat microspores. Plant Cell Tiss Organ Cult 122, 227–237 (2015). https://doi.org/10.1007/s11240-015-0763-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-015-0763-x