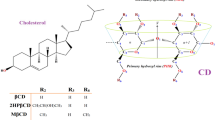
Abstract
Lipids being hydrophobic or amphiphilic can be encapsulated by cyclodextrin complexation. Among the various groups of lipids cholesterol, fatty acids, phospholipids and sphingolipids are overviewed concerning the structural requirements for both the lipid and the cyclodextrin component of the complexes. The chain length and the number and position of the double bonds in the fatty acids, the polarity of the head-group in the phospholipids and sphingolipids are important factors. Concerning the cyclodextrins, in addition to the most crucial cavity size also the chemical microenvironment of cavity entrances determine the interaction with lipids. While fatty acids, phospholipids and sphingolipids prefer the alpha-cyclodextrin cavity, cholesterol is complexed first of all by the beta-cyclodextrin and its derivatives. Methylated beta-cyclodextrin has extreme affinity to all of these lipids, which are common constituents of cell membranes. Based on the knowledge on the specific cyclodextrin-lipid interactions, cyclodextrin derivatives are able to selectively remove certain lipid components from model and biological membranes and can be selected making possible to modulate the lipid profile in such membranes.













Similar content being viewed by others
Abbreviations
- ACD:
-
Alpha-cyclodextrin
- ALA:
-
Alpha-linolenic acid
- BCD:
-
Beta-cyclodextrin
- BCFA:
-
Branched chain fatty acid
- CD:
-
Cyclodextrin
- CMC:
-
Critical micelle concentration
- DIMEB:
-
Dimethyl beta-cyclodextrin
- DHA:
-
Docosahexaenoic acid
- DLPC:
-
Dilauroyl phosphatidylcholine
- DMPC:
-
Dimyristoyl phosphatidylcholine
- DPPC:
-
Dipalmitoyl phosphatidylcholine
- DS:
-
Degree of substitution
- DSPC:
-
Distearoyl phosphatidylcholine
- EPA:
-
Eicosapentaenoic acid
- FA:
-
Fatty acid
- HPBCD:
-
Hydroxypropyl beta-cyclodextrin
- GCD:
-
Gamma-cyclodextrin
- LBPA:
-
Lysobisphosphatidic acid
- LUV:
-
Large unilamellar vesicle
- PLPC:
-
Palmitoyl-l-α-lysophosphatidylcholine
- POPC:
-
1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidylcholine
- PC:
-
Phosphatidylcholines
- PE:
-
Phosphatidylethanolamines
- PI:
-
Phosphoinositides
- PS:
-
Phosphatidylserines
- PUFA:
-
Polyunsaturated fatty acid
- RAMEA:
-
Randomly methylated alpha-cyclodextrin
- RAMEB:
-
Randomly methylated beta-cyclodextrin
- RAMEG:
-
Random methylated gamma-cyclodextrin
- SBEACD:
-
Sulfobutylether alpha-cyclodextrin
- SBEBCD:
-
Sulfobutylated beta-cyclodextin
- SBEGCD:
-
Sulfobutylated gamma-cyclodextrin
- SPM:
-
Sphingomyelins
- SUV:
-
Small unilamellar vesicle
- TRIMEA:
-
Hexakis(2,3,6-tri-O-methyl) alpha-cyclodextrin
- TRIMEB:
-
Heptakis(2,3,6-tri-O-methyl) beta-cyclodextrin
References
Cermenati G, Mitro N, Audano M, Melcangi RC, Crestani M, De Fabiani E, Caruso D (2015) Lipids in the nervous system: from biochemistry and molecular biology to patho-physiology. Biochim Biophys Acta 1851(1):51–60
Cermenati G, Abbiati F, Cermenati S, Brioschi E, Volonterio A, Cavaletti G, Saez E, De Fabiani E, Crestani M, Garcia-Segura LM, Melcangi RC, Caruso D, Mitro N (2012) Diabetes-induced myelin abnormalities are associated with an altered lipid pattern: protective effects of LXR activation. J Lipid Res 53(2):300–310
Szejtli J (2015) Ubiquitous cyclodextrins. In: Hargittai B, Hargittai I (eds) Culture of Chemistry: The Best Articles on the Human Side of 20th-Century Chemistry from the Archives of the Chemical Intelligencer. Springer Science + Business Media, New York, pp. 261–269
Fenyvesi É, Vikmon M, Szente L (2016) Cyclodextrins in food technology and human nutrition: benefits and limitations. Crit Rev Food Sci Nut 56(12):1981–2004
Uekaji Y, Jo A, Ohnishi M, Nakata D, Terao K (2012) A new generation of nutra-ceuticals and cosme-ceuticals complexing lipophilic bioactives with gamma-cyclodextrin. Transact Mater Res Soc Japan 37(1):89–94
Hood RL, Oakenfull DG, Sidhu GS (1995) Fat-modified eggs: nutritional and technological aspects. In: Ong ASH, Niki E, Packer L (eds) Nutrition, Lipids, Health, and Disease. AOCS Press, Champaigne, Illinois
Comerford KB, Artis JD, Jen KLC, Karakas SE (2011) The beneficial effects alpha-cyclodextrin on blood lipids and weight loss in healthy humans. Obesity 19:1200–1204
Vance JE, Karten B (2014) Thematic review series: recent advances in the treatment of lysosomal storage diseases. Niemann-Pick C disease and mobilization of lysosomal cholesterol by cyclodextrin. J Lipid Res 55(8):1609–1621
Yao J, Ho D, Calingasan NY, Pipalia NH, Lin MT, Beal MF (2012) Neuroprotection by cyclodextrin in cell and mouse models of Alzheimer disease. J Exp Med 209:2501–2513
Irie T, Otagiri M, Sunada M, Uekama K, Ohtani Y, Yamada Y, Sugiyama Y (1982) Cyclodextrin -induced hemolysis and shape changes of human erythrocytes in vitro. J Pharmacobio-Dyn 5(9):741–744
Miyajima K, Saito H, Nakagaki M (1987) Interaction of cyclodextrins with lipid membrane. Nippon Kagaku Kaishi 3:306 (Chem Abstr 107:73075)
Ravichandran R, Divakar S (1998) Inclusion of ring A of cholesterol inside the .beta.-cyclodextrin cavity: evidence from oxidation reactions and structural studies. J Incl Phenom Mol Recognit Chem 30(3):253–270
Köhler JEH, Hohla M, Sollner R, Amann M (1998) The difference between cholesterol- and glycyrrhizin-γ-cyclodextrin complexes - an analysis by MD simulations in vacuo and in aquo and the calculation of solvation free energies with AMSOL. Supramol Sci 5(1-2):117–137
Claudy P, Letoffe JM, Germain P, Bastide JP, Bayol A, Blasquez S, Rao RC, Gonzalez B Physicochemical characterization of cholesterol-beta cyclodextrin inclusion complexes. J Therm Anal 37(11-12):2497–2506
Frömming KH, Fridrich R, Mehnert W (1993) Inclusion compounds of cholesterol and β-cyclodextrin. Eur J Pharm Biopharm 39(4):148–152
dos Santos C, Buera MP, Mazzobre MF (2011) Phase solubility studies and stability of cholesterol/beta- cyclodextrin inclusion complexes. J Sci Food Agric 91(24):2551–2557
Higuchi T, Connors KA (1965) Phase-solubility techniques. Adv Anal Chem Instrum 4:117–212
Davidson CD, Fishman YI, Puskás I, Szemán J, Sohajda T, McCauliff LA, Sikora J, Storch J, Vanier MT, Szente L, Walkley SU, Dobrenis K (2016) Efficacy and ototoxicity of different cyclodextrins in niemann–pick c disease. Ann Clin Transl Neurol 3(5):366–380
Ishiguro T, Morishita E, Iohara D, Hirayama F, Wada K, Motoyama K, Arima H, Uekama K (2011) Some pharmaceutical and inclusion properties of 2-hydroxybutyl-beta- cyclodextrin derivative. Int J Pharm 419(1-2):161–169
Malanga M, Szemán J, Fenyvesi É, Puskás I, Csabai K, Gy G, Fenyvesi F, Szente L (2016) “Back to the future”: A new look at hydroxypropyl beta-cyclodextrins. J Pharm Sci 105(9):2921–2931
Loftsson T, Matthiasson K, Masson M (2003) The effects of organic salts on the cyclodextrin solubilization of drugs. Int J Pharm 262(1-2):101–107
Kiss T, Fenyvesi F, Bacskay I, Varadi J, Fenyvesi E, Ivanyi R, Szente L, Tosaki A, Vecsernyes M (2010) Evaluation of the cytotoxicity of beta-cyclodextrin derivatives: Evidence for the role of cholesterol extraction. Eur J Pharm Sci 40:376–380
Piel G, Piette M, Barillaro V, Castagne D, Evrard B, Delattre L (2007) Study of the relationship between lipid binding properties of cyclodextrins and their effect on the integrity of liposomes. Int J Pharm 338(1-2):35–42
Fenyvesi É, Szemán J, Csabai K, Malanga M, Szente L (2014) Methyl-beta-cyclodextrins: the role of number and types of substituents in solubilizing power. J Pharm Sci 103:1443–1452
Mascetti J, Castano S, Cavagnat D, Desbat B (2008) Organization of β-cyclodextrin under pure cholesterol, DMPC, or DMPG and mixed cholesterol/phospholipid monolayers. Langmuir 24:9616–9622
Lopez CA, de Vries AH, Marrink SJ (2013) Computational microscopy of cyclodextrin mediated cholesterol extraction from lipid model membranes. Sci Reports 3:2071
Zidovetzki R, Levitan I (2007) Use of cyclodextrins to manipulate plasma membrane cholesterol content: Evidence, misconceptions and control strategies. Biochim Biophys Acta 1768:1311–1324
Ohvo H, Slotte JP (1996) Cyclodextrin-mediated removal of sterols from monolayers: effects of sterol structure and phospholipids on desorption rate. Biochemist 35(24):8018–8024
Niu SL, Litman BJ (2002) Determination of membrane cholesterol partition coefficient using a lipid vesicle–cyclodextrin binary system: effect of phospholipid acyl chain unsaturation and headgroup composition. Biophys J 83:3406–3415
Gutay-Tóth Z, Fenyvesi F, Bársony O, Szente L, Goda K, Szabó G, Bacsó Z (2016) Cholesterol-dependent conformational changes of p-glycoprotein are detected by the 15d3 monclonal antibody. Biochim Biophys Acta – Mol Cell Biol Lipids 1861(3):188–195
Irie T, Fukunaga K, Garwood MK, Carpenter TO, Pitha J, Pitha J (1992) Hydroxypropyl cyclodextrins in parenteral use. II: Effects on transport and disposition of lipids in rabbit and humans. J Pharm Sci 81(6):524–528
Christian AE, Haynes MP, Phillips MC, Rothblat GH (1997) Use of cyclodextrins for manipulating cellular cholesterol content, J. Lipid Res 38:2264–2272
Christian AE, Byun HS, Zhong N, Wanunu M, Marti T, Fürer A, Diederich F, Bittman R, Rothblat GH (1999) Comparison of the capacity of β-cyclodextrin derivatives and cyclophanes to shuttle cholesterol between cells and serum lipoproteins. J Lipid Res 40:1475–1482
Maxfield FR, Tabas I (2005) Role of cholesterol and lipid organization in disease. Nature 438:612–621
Zimmer S, Grebe A, Bakke SS, Bode N, Halvorsen B, Ulas T, Skjelland M, De Nardo D, Labzin LI, Kerksiek A, Hempel C, Heneka MT, Hawxhurst V, Fitzgerald ML, Trebicka J, Björkhem I, Gustafsson J-Å, Westerterp M, Tall AR, Wright SD, Espevik T, Schultze JL, Nickenig G, Lütjohann D, Latz E (2016) Cyclodextrin promotes atherosclerosis regression via macrophage reprogramming. Sci Transl Med 8:333–350
Okamatsu A, Motoyama K, Onodera R, Higashi T, Koshigoe T, Shimada Y, Hattori K, Takeuchi T, Arima H (2013) Folate-appended β-cyclodextrin as a promising tumor targeting carrier for antitumor drugs in vitro and in vivo. Bioconjug Chem 24(4):724–733
Yokoo M, Kubota Y, Motoyama K, Higashi T, Taniyoshi M, Tokommaru H, Nishiyama R, Tabe Y, Mochinaga S, Sato A, Sueoka-Aragane N, Sueoka E, Arima H, Irie T, Kimura S (2015) 2-Hydroxypropyl-β-cyclodextrin acts as a novel anticancer agent. Plos One 10(11):e0141946
Yao J, Ho D, Calingasan NY, Pipalia NH, Lin MT, Beal MF (2012) Neuroprotection by cyclodextrin in cell and mouse models of. Alzheimer disease J Exp Med. 209(13):2501–2513
Liu B (2012) Therapeutic potential of cyclodextrins in the treatment of Niemann–Pick type C disease. Clin Lipidol 7(3):289–301
Tanaka Y, Yamada Y, Ishitsuka Y, Matsuo M, Shiraishi K, Wada K, Uchio Y, Kondo Y, Takeo T, Nakagata N, Higashi T, Motoyama K, Arima H, Mochinaga S, Higaki K, Ohno K, Irie T (2015) Efficacy of 2-hydroxypropyl-β-cyclodextrin in Niemann-Pick Disease type C model mice and its pharmacokinetic analysis in a patient with the disease. Biol Pharm Bull 38(6):844–851
Camargo F, Erickson RP, Garve WS, Hossain GS, Carbone PN, Heidenreich RA, Blanchard J (2001) Cyclodextrins in the treatment of a mouse model of Niemann-Pick C disease. Life Sci 70(2):131–142
Schlenk W, Sand DM (1961) Association of alfa-, and beta-cyclodextrins with organic acids. J Am Chem Soc 83:2312–2320
Ishiguro T, Adachi S, Matsuno R (1995) Thermogravimetric analysis of cyclodextrin-fatty acid complex formation and its use for predicting suppressed autoxidation of fatty acids. Biosci Biotechnol Biochem 59(1):51–54
Mikuni K, Hara K, Qiong W, Hara K, Hashimoto H (1999) Oxidative stability of docosahexaenoic acid oil (triglyceride form) included in cyclodextrins. In: Labandeira JJ, Torres Vila-Jato JL (eds) Proc Int Symp Cyclodextrins, 9th, Meeting Date 1998. Kluwer Academic Publishers, Dordrecht, Neth, pp. 549–552
Matsui Y, Yoneyama T (1996) NMR spectroscopy on inclusion complexes of cyclodextrins with unsaturated fatty acids. 14th National Symposium on Cyclodextrins, September1996, Nagasaki, Japan
Okada Y, Koizumi K, Ogata K, Ohfuji T (1989) Inclusion complexes of lipids with branched cyclodextrins. Chem Pharm Bull 37(11):3096–3099
Reiners RA, Birkhaug FJ (1970) Glyceride treatment of oils for reduction of free fatty acid content. US Pat 3491132
Conte JA, Stauffer KR (1996) Free fatty acid removal from used frying fat. US 5560950
Shimada K, Kawano K, Ishii J, Nakamura T (1992) Structure of inclusion complexes of cyclodextrins with triglyceride at vegetable oil/water interface. J Food Sci 57:655–656
Szejtli J, Banky-Elod E, Stadler A, Tetenyi P, Hethelyi I, Kernoczy L (1979) Enrichment of the unsaturated components in fatty acid ester mixtures by cyclodextrin complex formation. Acta Chim Acad Sci Hung 99(4):447–452
Szejtli J (1984) Industrial Applications of Cyclodextrins. In: Inclusion Compounds Vol.3. Academic, London
Wang J, Zhang J-L, Wu F-A (2013) Enrichment process for alpha-linolenic acid from silkworm pupae oil. Eur J Lipid Sci Technol 115(7):791–799
Bru R, Lopez-Nicolas JM, Garcia-Carmona F (1995) Aggregation of polyunsaturated fatty acids in the presence of cyclodextrins. Colloids Surf A Physicochem Eng Asp 97(3):263–269
Jyothirmayi N, Ramadoss CS, Divakar S (1991) Nuclear magnetic resonance studies of cyclodextrin complexes of linoleic acid and arachidonic acid. J Agric Food Chem 39(12):2123–2127
Trichard L, Delgado-Charro MB, Guy RH, Fattal E, Bochot A (2008) Novel beads made of alpha-cyclodextrin and oil for topical delivery of a lipophilic drug. Pharm Res 25(2):435–440
Wacker Cemie Info Sheet: Cawamax 6 – Stable oil-in-water emulsionshttps://www.wacker.com/cms/media/publications/downloads/6917_EN.pdf. Accessed 22 Sept 2016
Regiert M, Wimmer T, Moldenhauer J-P (1996) Application of γ-cyclodextrin for the stabilization and/or dispersion of vegetable oils containing triglycerides of polyunsaturated acids. J Incl Phenom Mol Recognit Chem 25(1-3):213–216
Bo**ova T, Coppel Y, Lauth-de Viguerie N, Milius A, Rico- Lattes I, Lattes A (2003) Complexes between beta-cyclodextrin and aliphatic guests as new noncovalent amphiphiles: Formation and physicochemical studies. Langmuir 19(13):5233–5239
Young OA, Gupta RB, Sadooghy-Saraby S (2012) Effect of cyclodextrins on the flavor of goat milk and its yoghurt. J Food Sci 77:S122–S127
Miyamoto Y, Nakahara M, Motoyama K, Ishiguro T, Oda Y, Yamanoi T, Okamoto I, Yagi A, Nishimura H, Hirayama F, Uekama K, Arima H (2011) Improvement of some physicochemical properties of arundic acid, (R)-(-)-2-propyloctanonic acid, by complexation with hydrophilic cyclodextrins. Int J Pharm 413(1-2):63–72
Szente L, Szejtli J, Szeman J, Kato L (1993) Fatty acid-cyclodextrin complexes: properties and applications. J Incl Phenom Mol Recognit Chem 16(4):339–354
Rajnavölgyi É, Laczik R, Kun V, Szente L, Fenyvesi É (2014) Effects of RAMEA-complexed polyunsaturated fatty acids on the response of human dendritic cells to inflammatory signals. Beilstein J Org Chem 10:3152–3160
Lehninger DL, Nelson DL, Cox MM (2016) Principles of Biochemistry. Chapter 9. W.H. Freeman & Co, London http://www.bioinfo.org.cn/book/biochemistry/chapt09/sim1.htm . Accessed 22 Sept
Slotte JP, Illman S (1996) Desorption of fatty acids from monolayers at the air/water interface to .beta.-cyclodextrin in the subphase. Langmuir 12(23):5664–5668
Roldan-Assad R, Gareil P (1995) Capillary zone electrophoretic determination of C2-C18 linear saturated free fatty acids with indirect absorbance detection. J Chromatogr A 708(2):339–350
Parker KM, Stalcup AM (2008) Affinity capillary electrophoresis and isothermal titration calorimetry for the determination of fatty acid binding with beta-cyclodextrin. J Chromatogr A 1204(2):171–182
European Patent Office Espacenet Database 2016. https://worldwide.espacenet.com. Accessed 22 Sept
Yamane I, Kan M, Minamoto Y, Amatsuji Y (1981) α-Cyclodextrin, a novel substitute for bovine albumin in serum-free culture of mammalian cells. Proc Jpn Acad Ser B 57(10):385–389
Nakama A (1991) Utilization of cyclodextrin as fat soluble compound carrier to serum-free culture of rat astrocytes. Ann Rep Osaka City Inst Public Health Environ Sci 54:48–53 (Chem Abstr 96:100488)
Kato L, Szejtli J, Szente L Water-soluble complexes of palmitic acid and palmitates for metabolic studies and cultivation trials of mycobacterium, leprae. Int J Leprosy 60:105–107
Mouslim J, El Haloui N-E, David L (1997) Influence of fatty acids and detergents on polyether antibiotic production by Streptomyces hygroscopicus NRRL B-1865. Can J Microbiol 43(9):879–883
Imaizumi A, Suzuki Y, Ono S, Sato H, Sato Y (1983) Heptakis(2,6-O-dimethyl)-β- cyclodextrin: a novel growth stimulant for Bordetella pertussis phase I. J Clin Microbiol 17(5):781–786
Yamane I (1985) Culture medium. US Pat 4533637
Yoshii H, Furuta T, Yasunishi A, Linko Y-Y, Linko P (1996) Oxidation stability of eicosapentaenoic and docosahexaenoic acid included in cyclodextrins. J Incl Phenom Mol Recognit Chem 25(1-3):217–220
Wagu M, Hayashi S, Kodama K (1984) US Pat 4438106
Matsuda Y, Ootsuka M, Teraoka R (1995) Preparation of polyenoic acid inclusion compounds with improved solubility and bioavailability. JP Pat 07025816
Kobayashi K, Hamazaki K, Fujioka S, Terao K, Yamamoto J, Kobayashi S (2007) The effect of n-3 PUFA/gamma-cyclodextrin complex on serum lipids in healthy volunteers - a randomized, placebo-controlled, double-blind trial. Asia Pac J Clin Nutr 16(3):429–434
Artiss J, Jen C (2009) Composition comprising dietary fat complexer comprising alpha- cyclodextrin and lipase inhibitor, and methods of using same for promoting weight loss. US Pat Appl 20090023682.
Artiss JD, Brogan K, Brucal M, Moghaddam M, Jen KL (2005) The effects of a new soluble dietary fiber on weight gain and selected blood parameters in rats. Metabolism. Clin Exp 55:195–202
Miyajima K, Tomita K, Nakagaki M (1985) Complex formation between di- and monophosphatidylcholines and cyclodextrins in water. Chem Pharm Bull 33(6):2587–2590
Debouzy JC, Fauvelle F, Crouzy S, Chapron Y, Goschl M, Gadelle A (1998) Mechanism of .alpha.-cyclodextrin induced hemolysis. 2. A study of the factors controlling the association with serine-, ethanolamine-, and choline-phospholipids. J Pharm Sci 87(1):59–66
Ahmed SM, Casu B, Cedro A, Guerrini M, Lanzarotti E, Moltrasio D, Naggi A, Torri G (1994) Disruption of micellar aggregates of ganglioside GM-1 by complexation with α-cyclodextrin. Int J Pharm 109(2):99–106
Szejtli J, Cserhati T, Szogyi M (1986) Interactions between cyclodextrins and cell-membrane phospholipids. Carbohydr Polym 6(1):35–49
Kobayashi T, Beuchat MH, Lindsay M, Frias S, Palmiter RD, Sakuraba H, Parton RG, Gruenberg J (1999) Late endosomal membranes rich in lysobisphosphatidic acid regulate cholesterol transport. Nat Cell Biol 1(2):113–118
Singh I, Kishimoto Y (1983) Effect of cyclodextrins on the solubilization of lignoceric acid, ceramide, and cerebroside, and on the enzymatic reactions involving these compounds. J Lipid Res 24(5):662–665
Stryer L (1975) Biosynthesis of Macromolecular Precursors. In: Biochemsitry. W H Freeman & Co, London
Ermolinsky B, Peredelchuk M, Provenzano D (2013) α-Cyclodextrin decreases cholera toxin binding to GM(1)- gangliosides. J Med Microbiol 62:1011–1014
Mitsumori R, Kato T, Hatanaka K (2009) γ-Cyclodextrin increases hydrolysis of gangliosides by sialidase from Arthrobacter ureafaciens: hydrolysis of gangliosides. Int J Carbohydr Chem ID 398284. doi: 10.1155/2009/398284. Accessed 22 Sept 2016
Nishijo JSS, Mazima K, Inoue Y, Mizuno H, Yoshida J (2000) Interactions of cyclodextrins with dipalmitoyl, distearoyl, and dimyristoyl phosphatidyl choline liposomes. A study by leakage of carboxyfluorescein in inner aqueous phase of unilamellar liposomes. Chem Pharm Bull 48(1):48–52
Puskas I, Csempesz F (2007) Influence of cyclodextrins on the physical stability of dipalmitoyl phosphatidyl choline liposomes. Colloids Surf B: Biointerfaces 58(2):218–224
Anderson TG, Tan A, Ganz P, Seelig J (2004) Calorimetric measurement of phospholipid interaction with methyl-beta-cyclodextrin. Biochemist 43:2251–2261
Ohtani Y, Irie T, Uekama K, Fukunaga K, Pitha J (1989) Differential effects of α-, β- and γ-cyclodextrins on human erythrocytes. Eur J Biochem 186(1-2):17–22
Motoyama K, Arima H, Toyodome H, Irie T, Hirayama F, Uekama K (2006) Effect of 2,6-di-O-methyl-alpha-cyclodextrin on hemolysis and morphological change in rabbit’s red blood cells. Eur J Pharm Sci 29(2):111–119
Kainu V, Hermansson M, Somerharju P (2010) Introduction of phospholipids to cultured cells with cyclodextrin. J Lipid Res 51:3533–3541
Huang Z, London E (2013) Effect of cyclodextrin and membrane lipid structure upon cyclodextrin-lipid interaction. Langmuir 29:14631–14638
Fukasawa M, Nishijima M, Itabe H, Takano T, Hanada K (2000) Reduction of sphingomyelin level without accumulation of ceramide in Chinese hamster ovary cells affects detergent-resistant membrane domains and enhances cellular cholesterol efflux to methyl-β-cyclodextrin. J Biol Chem 275(44):34028–34034
Acknowledgment
The financial support of National Research Fund (Multidrug project, NKFP-1A-041/2004) and EU Framework 7 (Tornado project, EU FP7 KBBE-2007-2-2-07-222720) is greatly acknowledged. The authors thank Mr. Mihaly Balint for the graphical illustrations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Szente, L., Fenyvesi, É. Cyclodextrin-Lipid Complexes: Cavity Size Matters. Struct Chem 28, 479–492 (2017). https://doi.org/10.1007/s11224-016-0884-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-016-0884-9