Abstract
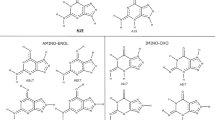
A heteroassociation of the antitumor antibiotic novatrone (NOV) and flavin mononucleotide (FMN) in aqueous solution was studied by one- and two-dimentional 1H NMR spectroscopy (500 MHz) to elucidate the molecular mechanism of the possible combined action of the antibiotic and the vitamin. The equilibrium reaction constants, the induced proton chemical shifts, and the thermodynamic parameters (ΔH and ΔS) of the NOV and FMN heteroassociation were determined from the concentration and temperature dependences of proton chemical shifts of the aromatic molecules. The most favorable structure of the 1 : 1 NOV-FMN complex was determined by both the method of molecular mechanics (X-PLOR software) and the induced proton chemical shifts of the molecules. An analysis of the results suggests that the NOV-FMN intermolecular complexes are mainly stabilized by stacking interactions of their aromatic chromophores. An additional stabilization is possible due to intermolecular hydrogen bonds. It was concluded that the aromatic molecules of vitamins, in particular, FMN, can form energetically favorable heterocomplexes with aromatic antitumor antibiotics in aqueous solutions, which could result in a modulation of their medical and biological action.
Similar content being viewed by others
Abbreviations
- FMN:
-
flavin momonucleotide
- NOV:
-
antibiotic novatrone
REFERENCES
Traganos, F., Kapuscinsky, J., and Darzynkiewicz, Z., Cancer Res., 1991, vol. 51, pp. 3682–3689.
Lyles, M.B. and Cameron, I.L., Biophys. Chem., 2002, vol. 96, pp. 53–76.
Piosik, J., Zdunek, M., and Kapuscinsky, J., Biochem. Pharmacol., 2002, vol. 63, pp. 635–646.
Davies, D.B., Veselkov, D.A., Djimant, L.N., and Veselkov, A.N., Eur. Biophys. J., 2001, vol. 30, pp. 354–366.
Birchall, L.A., Bailey, N.P., and Blackledge, G.R.P., Br. J. Clin. Pract., 1991, vol. 45, pp. 208–211.
Kapuscinsky, J. and Darzynkiewicz, Z., Biochem. Pharmacol., 1985, vol. 34, pp. 4203–4213.
Kapuscinsky, J. and Darzynkiewicz, Z., Proc. Natl. Acad. Sci. USA, 1986, vol. 83, pp. 6302–6306.
Roboz, J., Richardson, C.L., and Holland, J.F., Life Sci., 1982, vol. 31, pp. 25–30.
Veselkov, A.N., Evstigneev, M.P., Vysotskii, S.A., Veselkov, D.A., and Devis, D.B., Biofizika, 2002, vol. 47, pp. 459–466.
Pullman, B., Adv. Drug Res., 1989, vol. 18, pp. 2–112.
Armitage, J.O., Oncology, 2002, vol. 16, pp. 490–518.
Adel, A.L., Dorr, R.T., and Liddil, J.D., Cancer Invest., 1993, vol. 11, pp. 15–24.
Veselkov, A.N., Lantushenko, A.O., Chubarov, A.S., Veselkov, D.A., and Devis, D.B., Khim. Fiz., 2002, vol. 76, pp. 1313–1320.
Davies, D.B., Veselkov, D.A., and Veselkov, A.N., Mol. Phys., 1999, vol. 97, pp. 439–451.
Davies, D.B., Veselkov, D.A., Kodintsev, V.V., Evstigneev, M.P., and Veselkov, A.N., Mol. Phys., 2000, vol. 98, pp. 1961–1972.
Davies, D.B., Veselkov, D.A., Evstigneev, M.P., and Veselkov, A.N., J. Chem. Soc., Perkin Trans. 1, 2001, pp. 61–67.
Veselkov, D.A., Evstigneev, M.P., Kodintsev, V.V., Dymant, L.N., Devis, D.B., and Veselkov, A.N., Khim. Fiz., 2001, vol. 75, pp. 879–884.
Ross, R.D. and Subramanian, S., Biochemistry, 1981, vol. 20, pp. 3096–3102.
Record, M.T., Anderson, C.F., and Lohman, T.M., Quart. Rev. Biophys., 1978, vol. 11, pp. 103–178.
Brunger, A.T., X-PLOR, A System for X-PLOR Crystallography and NMR, Yale: Univ. Press, 1992, p. 382.
Jorgensen, W., Chaindrasekhar, J., Madura, J., Imprey, R., and Klein, M., J. Chem. Phys., 1983, vol. 79, pp. 926–935.
Kleywegt, G.L., Dictionaries for Heteros / News From Uppsala Software Factory. 5, 1998.
Berman, H.M., Westbrook, J., Feng, Z., Gilliland, G., Bhat, T.N., Weissig, H., Shindyalov, I.N., and Bourne, P.E., Nucleic Acids Res., 2000, vol. 28, pp. 235–242.
Allinger, N.L., J. Am. Chem. Soc., 1977, vol. 99, pp. 8127–8134.
Falk, M., Chew, W., Walter, J.A., Kwiatkowski, W., Barclay, K.D., and Klassen, G.A., Can. J. Chem., 1998, vol. 76, pp. 48–56.
Author information
Authors and Affiliations
Corresponding author
Additional information
__________
Translated from Bioorganicheskaya Khimiya, Vol. 31, No. 5, 2005, pp. 503–510.
Original Russian Text Copyright © 2005 by Veselkov, Evstigneev, Rozvadovska, Mukhina, Davies.
Rights and permissions
About this article
Cite this article
Veselkov, A.N., Evstigneev, M.P., Rozvadovska, A.O. et al. A Structural and Thermodynamic Analysis of Novatrone and Flavin Mononucleotide Heteroassociation in Aqueous Solution by 1H NMR Spectroscopy. Russ J Bioorg Chem 31, 453–459 (2005). https://doi.org/10.1007/s11171-005-0062-0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11171-005-0062-0