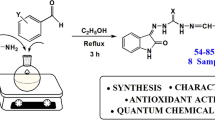
Abstract
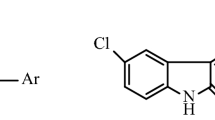
Five new Schiff bases of isatin and its derivatives were prepared from monothiocarbohydrazides and 5-chloro isatin. The chemical structures of the synthesized compounds were performed by 1H NMR, 13C NMR, and FT-IR spectroscopic techniques and elemental analysis. The in vitro antioxidant activities of all the products were determined by 1,1-Diphenyl-2-Picryl Hydrazyl free radical scavenging method. It also examined the antioxidant properties of the compounds based on quantum chemical calculations as well as supporting experimental spectroscopic data. Theoretical calculations carried out at B3LYP correlation functional with 6-311++g(2d,2p) basis set. Some chemical reactivity descriptors obtained from AIM, NCI, and ELF analysis were used to reveal the relationship between the electronic and antioxidant properties of the compounds. Furthermore, the bond lengths, charge densities, potential energy densities, inter-atomic dipole moments, and delocalization indices of the active phenolic hydrogen bonds of the compounds were shown to be parameters that can be used to determine the antioxidant properties of compounds.
Graphic Abstract
New β-isatin aldehyde-N,N′-thiocarbohydrazones were synthesized. Structures of synthesized molecules were clarified using spectroscopic methods. Antioxidant activities of the compounds were tested by the DPPH method. AIM, NCI, and ELF analysis were performed to investigate the relationship between the electronic properties and antioxidant activity.











Similar content being viewed by others
References
S.N. Pandeya, S. Smitha, M. Jyoti, S.K. Sridhar, Acta Pharm. 55, 27 (2005)
Z.H. Chohan, H. Pervez, A. Rauf, K.M. Khan, C.T. Supuran, J. Enzyme Inhib. Med. Chem. 19, 417 (2004)
S.Y. Abbas, A.A. Farag, Y.A. Ammar, A.A. Atrees, A.F. Mohamed, A.A. El-Henawy, Monat. Chem. 144, 1725 (2013)
A. Jarrahpour, D. Khalili, E. De Clercq, C. Salmi, J. Brunel, Molecules 12, 1720 (2007)
A.V. Bogdanov, I.F. Zaripova, A.D. Voloshina, A.S. Strobykina, N.V. Kulik, S.V. Bukharov, J.K. Voronina, A.R. Khamatgalimov, V.F. Mironov, Monat. Chem. 149, 111 (2018)
H. Muğlu, Res. Chem. Intermed. 46, 2083 (2020)
T.K. Bakır, J.B. Lawag, Res. Chem. Intermed. 46, 2541 (2020)
M. Verma, S.N. Pandeya, K.N. Singh, J.P. Stables, Acta Pharm. 54, 49 (2004)
T. Aboul-Fadl, F.A. Bin-Jubair, Int. J. Res. Pharm. Sci. 1, 113 (2010)
T.R. Bal, B. Anand, P. Yogeeswari, D. Sriram, Bioorg. Med. Chem. Lett. 15, 4451 (2005)
D. Sinha, A.K. Tiwari, S. Singh, G. Shukla, P. Mishra, H. Chandra, A.K. Mishra, Eur. J. Med. Chem. 43, 160 (2008)
S. Pandeya, D. Sriram, Acta Pharm. Turcica 40, 33 (1998)
D. Sriram, S. Pandeya, G. Nath, E. De Clercq, Arzneimittelforschung 50, 55 (2000)
M. Sathisha, V. Revankar, K. Pai, Met. Based Drugs 2008 (2008)
C. Liang, J. **a, D. Lei, X. Li, Q. Yao, J. Gao, Eur. J. Med. Chem. 74, 742 (2014)
M.T. Gabr, N.S. El-Gohary, E.R. El-Bendary, M.M. El-Kerdawy, N. Ni, Eur. J. Med. Chem. 128, 36 (2017)
G. Kiran, M. Sarangapani, T. Gouthami, A.R. Narsimha Reddy, Toxicol. Environ. Chem. 95, 367 (2013)
K. Gangarapu, S. Manda, A. Jallapally, S. Thota, S.S. Karki, J. Balzarini, E. De Clercq, H. Tokuda, Med. Chem. Res. 23, 1046 (2014)
P. Wanasundara, F. Shahidi, Antioxidants: Science, Technology, and Applications, 6th edn. (Wiley Interscience, Hoboken, 2005)
G. Kiran, T. Maneshwar, Y. Rajeshwar, M. Sarangapani, J. Chem. 2013 (2013)
M. Premanathan, S. Radhakrishnan, K. Kulangiappar, G. Singaravelu, V. Thirumalaiarasu, T. Sivakumar, K. Kathiresan, Indian J. Med. Res. 136, 822 (2012)
A.I. Elshamy, T. Yoneyama, N. Van Trang, N.T. Son, Y. Okamoto, S. Ban, M. Noji, A. Umeyama, J. Mol. Struct. 1200, 127061 (2020)
N.T. Son, D.T.M. Thanh, N. Van Trang, J. Mol. Struct. 1193, 76 (2019)
T.S. Ahamed, V.K. Rajan, K. Sabira, K. Muraleedharan, Comput. Biol. Chem. 80, 66 (2019)
S. Yu, Y. Wang, Y. Ma, L. Wang, J. Zhu, S. Liu, Inorg. Chim. Acta 468, 159 (2017)
W. Brand-Williams, M.-E. Cuvelier, C. Berset, LWT-Food Sci. Technol. 28, 25 (1995)
D. Huang, B. Ou, R.L. Prior, J. Agric. Food Chem. 53, 1841 (2005)
S. Mukherjee, N. Pawar, O. Kulkarni, B. Nagarkar, S. Thopte, A. Bhujbal, P. Pawar, BMC Complement. Altern. Med. 11, 38 (2011)
W. Kohn, L.J. Sham, Phys. Rev. 140, A1133 (1965)
P. Hohenberg, W. Kohn, Phys. Rev. 136, B864 (1964)
M. Frisch, G. Trucks, H. Schlegel, G. Scuseria, M. Robb, J. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. Petersson, D. Fox, Wallingford (2010)
R. Bader, Atoms in Molecule (Oxford University Press, Oxford, 1990)
A.E. Reed, L.A. Curtiss, F. Weinhold, Chem. Rev. 88, 899 (1988)
J. Carpenter, F. Weinhold, J. Mol. Struct: Theochem 169, 41 (1988)
A.E. Reed, F. Weinhold, J. Chem. Phys. 78, 4066 (1983)
A.E. Reed, R.B. Weinstock, F. Weinhold, J. Chem. Phys. 83, 735 (1985)
R.F. Bader, Chem. Rev. 91, 893 (1991)
R.F. Bader, Acc. Chem. Res. 18, 9 (1985)
V.V. Dabholkar, D.R. Tripathi, Indian J. Chem. Sec. B 49B, 593 (2010)
Z. Shi, Z. Zhao, M. Liu, X. Wang, Comptes Rendus Chim. 16, 977 (2013)
A. Abdel-Aziem, B.S. Baaiu, A.O. Abdelhamid, J. Heterocycl. Chem. 54, 3471 (2017)
O. Bekircan, H. Bektas, Molecules 13, 2126 (2008)
I. Fleming, D.H. Williams, Spectroscopic Methods in Organic Chemistry (McGraw-Hill, New York, 1966)
N. Naik, H. Vijay Kumar, P.B. Vidyashree, J. Pharm. Res. 4, 2686 (2011)
A. Božić, N. Filipović, I. Novakovic, S. Bjelogrlić, J. Nikolić, S. Drmanić, A. Marinković, J. Serb. Chem. Soc. 82, 495 (2017)
G. Sammaiah, G. Brahmeshwari, M. Sarangapani, J. Adv. Pharm. Sci. 1, 47 (2011)
A. Andreani, S. Burnelli, M. Granaiola, A. Leoni, A. Locatelli, R. Morigi, M. Rambaldi, L. Varoli, M.A. Cremonini, G. Placucci, Eur. J. Med. Chem. E 45, 1374 (2010)
F. Shahidi, H.J. Zhong, P. Ambigaipalan, Bailey’s industrial oil and fat products 6, 491 (2005)
F. Shahidi, Y. Zhong, J. Agric. Food Chem. 59, 8 (2011)
M.S. Çavuş, H. Yakan, H. Muğlu, T. Bakır, J. Phys. Chem. Solids 140, 109362 (2020)
H. Muğlu, M.S. Çavuş, T. Bakır, H. Yakan, J. Mol. Struct. 1196, 819 (2019)
J. Chen, J. Yang, L. Ma, J. Li, N. Shahzad, C.K. Kim, Sci. Rep. 10(1), 2611 (2020)
J.-C. Cheng, F. Dai, B. Zhou, L. Yang, Z.-L. Liu, Food Chem. 104, 132–139 (2007)
M.I. de Heer, P. Mulder, H.-G. Korth, K.U. Ingold, J. Lusztyk, J. Am. Chem. Soc. 122, 2355–2360 (2000)
R. Farhoosh, S. Johnny, M. Asnaashari, N. Molaahmadibahraseman, A. Sharif, Food Chem. 194, 128 (2016)
J.S. Wright, E.R. Johnson, G.A. DiLabio, J. Am. Chem. Soc. 123, 1173 (2001)
Author information
Authors and Affiliations
Contributions
HY was involved in synthesis, structure elucidation, and writing-original draft preparation. HM was involved in synthesis, methodology, and structure elucidation. TKB was involved in antioxidant activity studies and writing-original draft preparation. MSÇ was involved in density functional theory calculations and writing-original draft preparation.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yakan, H., Bakır, T.K., Çavuş, M.S. et al. New β-isatin aldehyde-N,N′-thiocarbohydrazones: preparation, spectroscopic studies and DFT approach to antioxidant characteristics. Res Chem Intermed 46, 5417–5440 (2020). https://doi.org/10.1007/s11164-020-04270-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-020-04270-0