Abstract
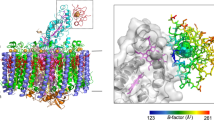
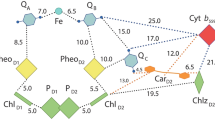
The photosynthetic reaction centers (RCs) classified as the group II possess a peripheral cytochrome (Cyt) subunit, which serves as the electron mediator to the special-pair. In the cycle of the photosynthetic electron transfer reactions, the Cyt subunit accepts electrons from soluble electron carrier proteins, and re-reduces the photo-oxidized special-pair of the bacteriochlorophyll. Physiologically, high-potential cytochromes such as the cytochrome c2 and the high-potential iron–sulfur protein (HiPIP) function as the electron donors to the Cyt subunit. Most of the Cyt subunits possess four heme c groups, and it was unclear which heme group first accepts the electron from the electron donor. The most distal heme to the special-pair, the heme-1, has a lower redox potential than the electron donors, which makes it difficult to understand the electron transfer mechanism mediated by the Cyt subunit. Extensive mutagenesis combined with kinetic studies has made a great contribution to our understanding of the molecular interaction mechanisms, and has demonstrated the importance of the region close to the heme-1 in the electron transfer. Moreover, crystallographic studies have elucidated two high-resolution three-dimensional structures for the RCs containing the Cyt subunit, the Blastochloris viridis and Thermochromatium tepidum RCs, as well as the structures of their electron donors. An examination of the structural data also suggested that the binding sites for both the cytochrome c2 and the HiPIP are located adjacent to the solvent-accessible edge of the heme-1. In addition, it is also indicated by the structural and biochemical data that the cytochrome c2 and the HiPIP dock with the Cyt subunit by different mechanisms although the two electron donors utilize the same region for the interactions; cytochrome c2 is recognized through electrostatic interactions while hydrophobic interactions are important in the HiPIP docking.
Similar content being viewed by others
References
N Adir HL Axelrod P Beroza RA Isaacson SH Rongey MY Okamura G Feher (1996) ArticleTitleCo-crystallization and characterization of the photosynthetic reaction center–cytochrome c2 complex from Rhodobacter sphaeroides Biochemistry 35 2535–2547
J Alric M Yoshida KV Nagashima R Hienerwadel P Parot A Vermeglio S-W Chen P J-L (2004) ArticleTitleTwo distinct binding sites for HiPIP and cytochrome c on the reaction center-bound cytochrome of Rubrivivax gelatinosus J Biol Chem 279 545–553
B Arnoux A Ducruix F Reiss-Husson M Lutz J Norris M Schiffer CH Chang (1989) ArticleTitleStructure of spheroidene in the photosynthetic reaction center from Y Rhodobacter sphaeroides FEBS Lett 258 47–50
HL Axelrod G Feher JP Allen AJ Chirino MW Day BT Hsu DC Rees (1994) ArticleTitleCrystallization and X-ray structure determination of cytochrome c2 from Rhodobacter sphaeroides in three crystal forms Acta Crystallogr D Biol Crystallogr D 50 596–602
HL Axelrod EC Abresch MY Okamura AP Yeh DC Rees G Feher (2002) ArticleTitleX-ray structure determination of the cytochrome c2: reaction center electron transfer complex from Rhodobacter sphaeroides J Mol Biol 319 501–515
RG Bartsch (1991) ArticleTitleThe distribution of soluble metallo-redox proteins in purple phototrophic bacteria Biochim Biophys Acta 1058 28–30
MM Benning G Wesenberg MS Caffrey RG Bartsch TE Meyer MA Cusanovich I Rayment HM Holden (1991) ArticleTitleMolecular structure of cytochrome c2 isolated from Rhodobacter capsulatus determined at 2.5 Å resolution J Mol Biol 220 673–685
MM Benning TE Meyer HM Holden (1994) ArticleTitleX-Ray structure of the cytochrome c2 isolated from Paracoccus denitrificans refined to 1.7-Å resolution Arch Biochem Biophys 310 460–466
MM Benning TE Meyer I Rayment HM Holden (1994) ArticleTitleMolecular structure of the oxidized high-potential iron–sulfur protein isolated from Ectothiorhodospira vacuolata Biochemistry 33 2476–2483
MM Benning TE Meyer HM Holden (1996) ArticleTitleMolecular structure of a high potential cytochrome c2 isolated from Rhodopila globiformis Arch Biochem Biophys 333 338–348
DR Breiter TE Meyer I Rayment HM Holden (1991) ArticleTitleThe molecular structure of the high potential iron–sulfur protein isolated from Ectothiorhodospira halophila determined at 2.5-Å resolution J Biol Chem 266 18660–18667
A Camara-Artigas JC Williams JP Allen (2001) ArticleTitleStructure of cytochrome c2 from Rhodospirillum centenum Acta Crystallogr D Biol Crystallogr 57 1498–1505
CW Carter SuffixJr (2001) High potential iron sulfur proteins A Messerschmidt R Huber T Poulos K Wieghardt (Eds) Handbook of Metalloproteins Wiley Chichester, UK 602–609
CW Carter SuffixJr J Kraut ST Freer X Nguyen Huu RA Alden RG Bartsch (1974) ArticleTitleTwo-Angstrom crystal structure of oxidized Chromatium high potential iron protein J Biol Chem 249 4212–4225
CH Chang O el-Kabbani D Tiede J Norris M Schiffer (1991) ArticleTitleStructure of the membrane-bound protein photosynthetic reaction center from Rhodobacter sphaeroides Biochemistry 30 5352–5360
IP Chen P Mathis J Koepke H Michel (2000) ArticleTitleUphill electron transfer in the tetraheme cytochrome subunit of the Rhodopseudomonas viridis photosynthetic reaction center: evidence from site-directed mutagenesis Biochemistry 39 3592–3602
AJ Chirino EJ Lous M Huber JP Allen CC Schenck ML Paddock G Feher DC Rees (1994) ArticleTitleCrystallographic analyses of site-directed mutants of the photosynthetic reaction center from Rhodobacter sphaeroides Biochemistry 33 4584–4593
MM Couture M Auger F Rosell AG Mauk E Boubour RB Lennox LD Eltis (1999) ArticleTitleInvestigation of the role of a surface patch in the self-association of Chromatium vinosum high potential iron–sulfur protein Biochim Biophys Acta 1433 159–169
H De Klerk MD Kamen (1966) ArticleTitleA high-potential non-haem iron protein from the facultative photoheterotrophe Rhodopseudomonas gelatinosa Biochim Biophys Acta 112 175–178
J Deisenhofer H Michel (1989) ArticleTitleThe photosynthetic reaction center from the purple bacterium Rhodopseudomonas vridis EMBO J 8 2149–2170
J Deisenhofer O Epp K Miki R Huber H Michel (1984) ArticleTitleX-ray structure analysis of a membrane protein complex Electron density map at 3 IssueIDthinsp;Å resolution and a model of the chromophores of the photosynthetic reaction center from Rhodopseudomonas viridis. J Mol Biol 180 385–398
J Deisenhofer O Epp K Miki R Huber H Michel (1985) ArticleTitleStructure of the protein subunits in the photosynthetic reaction center of Rhodopseudomonas viridis at 3 Å resolution Nature (London). 318 618–624
J Deisenhofer O Epp I Sinning H Michel (1995) ArticleTitleCrystallographic refinement at 2.3 Å resolution and refined model of the photosynthetic reaction centre from Rhodopseudomonas viridis J Mol Biol 246 429–457
SM Dracheva LA Drachev AA Konstantinov A Semenov VP Skulachev AM Arutjunjan VA Shuvalov SM Zaberezhnaya (1988) ArticleTitleElectrogenic steps in the redox reactions catalyzed by photosynthetic reaction-centre complex from Rhodopseudomonas viridis Eur J Biochem 171 253–264
K Dus H De Klerk K Sletten RG Bartsch (1967) ArticleTitleChemical characterization of high potential iron proteins from Chromatium and Rhodopseudomonas gelatinosa Biochim Biophys Acta 140 291–311
U Ermler G Fritzsch SK Buchanan H Michel (1994) ArticleTitleStructure of the photosynthetic reaction centre from Rhodobacter sphaeroides at 2.65 Å resolution: cofactors and protein-cofactor interactions Structure 2 925–936
A Fukushima K Matsuura K Shimada T Satoh (1988) ArticleTitleReaction center-B870 pigment protein complexes with bound cytochrome- c-555 and cytochrome- c-551 from Rhodocyclus gelatinosus Biochim Biophys Acta 933 399–405
G Garau S Geremia L Randaccio L Vaccari MS Viezzoli (2000) ArticleTitleCrystallization and preliminary X-ray analysis of two pH-dependent forms of cytochrome c2 from Rhodopseudomonas palustris Acta Crystallogr D Biol Crystallogr 56 IssueIDPt 12 1699–1701
A Gonzalez S Benini S Ciurli (2003) ArticleTitleStructure of Rhodoferax fermentans high-potential iron–sulfur protein solved by MAD Acta Crystallogr D Biol Crystallogr 59 1582–1588
A Hochkoeppler D Zannoni S Ciurli TE Meyer MA Cusanovich G Tollin (1996) ArticleTitleKinetics of photo-induced electron transfer from high-potential iron–sulfur protein to the photosynthetic reaction center of the purple phototroph Rhodoferax fermentans Proc Natl Acad Sci USA 93 6998–7002
CA Kerfeld AE Salmeen TO Yeates (1998) ArticleTitleCrystal structure and possible dimerization of the high-potential iron–sulfur protein from Chromatium purpuratum Biochemistry 37 13911–13917
DB Knaff A Willie JE Long A Kriauciunas B Durham F Millett (1991) ArticleTitleReaction of cytochrome c2 with photosynthetic reaction centers from Rhodopseudomonas viridis Biochemistry 30 1303–1310
PJ Kraulis (1991) ArticleTitleMolscript – a program to produce both detailed and schematic plots of protein structures J Appl Crystallogr 24 946–950
L Liu T Nogi M Kobayashi T Nozawa K Miki (2002) ArticleTitleUltrahigh-resolution structure of high-potential iron–sulfur protein from Thermochromatium tepidum Acta Crystallogr D Biol Crystallogr 58 1085–1091
K Matsuura (1994) ArticleTitleComparative and evolutionary aspects of the photosynthetic electron-transfer system of purple bacteria J Plant Res 107 191–200
K Matsuura K Shimada (1990) Evolutionary relationships between reaction center complexes with and without cytochrome c subunits in purple bacteria M Baltscheffsky (Eds) Current Research in Photosynthesis Kluwer Academic Dordrecht 193–196
K Matsuura A Fukushima K Shimada T Satoh (1988) ArticleTitleDirect and indirect electron-transfer from cytochrome- c and cytochrome- c2 to the photosynthetic reaction center in pigment-protein complexes isolated from Rhodocyclus gelatinosus FEBS Lett 237 21–25
L Menin B Schoepp P Parot A Vermeglio (1997) ArticleTitlePhotoinduced cyclic electron transfer in Rhodocyclus tenuis cells: participation of HiPIP or cyt c8 depending on the ambient redox potential Biochemistry 36 12183–12188
L Menin M Yoshida M Jaquinod KV Nagashima K Matsuura P Parot A Vermeglio (1999) ArticleTitleDark aerobic growth conditions induce the synthesis of a high midpoint potential cytochrome c8 in the photosynthetic bacterium Rubrivivax gelatinosus Biochemistry 38 15238–15244
EA Merritt DJ Bacon (1997) Raster3D: Photorealistic molecular graphics CW Carter RM Sweet (Eds) Methods in Enzymology Academic Press San Diego, CA 505–524
TE Meyer RG Bartsch MA Cusanovich G Tollin (1993) ArticleTitleKinetics of photooxidation of soluble cytochromes, Hipip, and Azurin by the photosynthetic reaction center of the purple phototrophic bacterium Rhodopseudomonas viridis Biochemistry 32 4719–4726
K Miki S Sogabe (2001) Cytochrome c2 A Messerschmidt R Huber T Poulos K Wieghardt (Eds) Handbook of Metalloproteins Wiley Chichester, UK 55–68
JM Moulis N Scherrer J Gagnon E Forest Y Petillot D Garcia (1993) ArticleTitlePrimary structure of Chromatium tepidum high-potential iron–sulfur protein in relation to thermal denaturation Arch Biochem Biophys 305 186–192
KV Nagashima K Shimada K Matsuura (1996) ArticleTitleShortcut of the photosynthetic electron transfer in a mutant lacking the reaction center-bound cytochrome subunit by gene disruption in a purple bacterium, Rubrivivax gelatinosus FEBS Lett 385 209–213
KVP Nagashima Y Sakuragi K Shimada K Matsuura (1998) ArticleTitleComparative analysis of the primary structure of the reaction center-bound cytochrome subunit in purple bacteria Photosynth Res 55 349–355
A Nicholls KA Sharp B Honig (1991) ArticleTitleProtein folding and association – insights from the interfacial and thermodynamic properties of hydrocarbons Proteins-Struct Func Genet 11 281–296
T Nogi I Fathir M Kobayashi T Nozawa K Miki (2000) ArticleTitleCrystal structures of photosynthetic reaction center and high-potential iron–sulfur protein from Thermochromatium tepidum: thermostability and electron transfer Proc Natl Acad Sci USA 97 13561–13566
T Nogi M Kobayashi T Nozawa K Miki (2000) ArticleTitleCrystallization and preliminary crystallographic analysis of the high-potential iron–sulfur protein from Thermochromatium tepidum Acta Crystallogr D Biol Crystallogr 56 656–658
JM Ortega P Mathis (1992) ArticleTitleEffect of temperature on the kinetics of electron transfer from the tetraheme cytochrome to the primary donor in Rhodopseudomonas viridis FEBS Lett 301 45–48
JM Ortega P Mathis (1993) ArticleTitleElectron transfer from the tetraheme cytochrome to the special pair in isolated reaction centers of Rhodopseudomonas viridis Biochemistry 32 1141–1151
JM Ortega F Drepper P Mathis (1999) ArticleTitleElectron transfer between cytochrome c2 and the tetraheme cytochrome c in Rhodopseudomonas viridis Photosynth Res 59 147–157
A Osyczka KV Nagashima S Sogabe K Miki M Yoshida K Shimada K Matsuura (1998) ArticleTitleInteraction site for soluble cytochromes on the tetraheme cytochrome subunit bound to the bacterial photosynthetic reaction center mapped by site-directed mutagenesis Biochemistry 37 11732–11744
A Osyczka KV Nagashima K Shimada K Matsuura (1999) ArticleTitleInteraction site for high-potential iron–sulfur protein on the tetraheme cytochrome subunit bound to the photosynthetic reaction center of Rubrivivax gelatinosus Biochemistry 38 2861–2865
A Osyczka KV Nagashima S Sogabe K Miki K Shimada K Matsuura (1999) ArticleTitleComparison of the binding sites for high-potential iron–sulfur protein and cytochrome c on the tetraheme cytochrome subunit bound to the bacterial photosynthetic reaction center Biochemistry 38 15779–15790
A Osyczka KV Nagashima S Sogabe K Miki K Shimada K Matsuura (2001) ArticleTitleDifferent mechanisms of the binding of soluble electron donors to the photosynthetic reaction center of Rubrivivax gelatinosus and Blastochloris viridis J Biol Chem 276 24108–24112
CC Page CC Moser X Chen PL Dutton (1999) ArticleTitleNatural engineering principles of electron tunnelling in biological oxidation–reduction Nature 402 47–52
E Parisini F Capozzi P Lubini V Lamzin C Luchinat GM Sheldrick (1999) ArticleTitleAb initio solution and refinement of two high-potential iron protein structures at atomic resolution Acta Crystallogr D Biol Crystallogr 55 1773–1784
MM Pereira AM Antunes OC Nunes MS Costa Particleda M Teixeira (1994) ArticleTitleA membrane-bound HIPIP type center in the thermohalophile Rhodothermus marinus FEBS Lett 352 327–330
MM Pereira JN Carita M Teixeira (1999) ArticleTitleMembrane-bound electron transfer chain of the thermohalophilic bacterium Rhodothermus marinus: characterization of the iron–sulfur centers from the dehydrogenases and investigation of the high-potential iron–sulfur protein function by in␣vitro reconstitution of the respiratory chain Biochemistry 38 1276–1283
MM Pereira JN Carita M Teixeira (1999) ArticleTitleMembrane-bound electron transfer chain of the thermohalophilic bacterium Rhodothermus marinus: a novel multihemic cytochrome bc, a new complex III Biochemistry 38 1268–1275
MM Pereira M Santana CM Soares J Mendes JN Carita AS Fernandes M Saraste MA Carrondo M Teixeira (1999) ArticleTitleThe caa3 terminal oxidase of the thermohalophilic bacterium Rhodothermus marinus: a HiPIP:oxygen oxidoreductase lacking the key glutamate of the D-channel Biochim Biophys Acta 1413 1–13
I Rayment G Wesenberg TE Meyer MA Cusanovich HM Holden (1992) ArticleTitleThree-dimensional structure of the high-potential iron–sulfur protein isolated from the purple phototrophic bacterium Rhodocyclus tenuis determined and refined at 1.5 Å resolution J Mol Biol 228 672–686
FR Salemme ST Freer NH Xuong RA Alden J Kraut (1973) ArticleTitleThe structure of oxidized cytochrome c2 of Rhodospirillum rubrum J Biol Chem 248 3910–3921
B Schoepp P Parot L Menin J Gaillard P Richaud A Vermeglio (1995) ArticleTitleIn vivo participation of a high potential iron–sulfur protein as electron donor to the photochemical reaction center of Rubrivivax gelatinosus Biochemistry 34 11736–11742
RJ Shopes LMA Levine D Holten CA Wraight (1987) ArticleTitleKinetics of oxidation of the bound cytochromes in reaction centers from Rhodopseudomonas viridis Photosynth Res 12 165–180
S Sogabe K Miki (1995) ArticleTitleRefined crystal structure of ferrocytochrome c2 from Rhodopseudomonas viridis at 1.6 Å resolution J Mol Biol 252 235–247
M Tetreault M Cusanovich T Meyer H Axelrod MY Okamura (2002) ArticleTitleDouble mutant studies identify electrostatic interactions that are important for docking cytochrome c2 onto the bacterial reaction center Biochemistry 41 5807–5815
G Driessche ParticleVan I Vandenberghe B Devreese B Samyn TE Meyer R Leigh MA Cusanovich RG Bartsch U Fischer JJ Van Beeumen (2003) ArticleTitleAmino acid sequences and distribution of high-potential iron–sulfur proteins that donate electrons to the photosynthetic reaction center in phototropic proteobacteria J Mol Evol 57 181–199
G Venturoli MD Mamedov SS Mansy F Musiani M Strocchi F Francia AY Semenov JA Cowan S Ciurli (2004) ArticleTitleElectron transfer from HiPIP to the photooxidized tetraheme cytochrome subunit of Allochromatium vinosum reaction center: new insights from site-directed mutagenesis and computational studies Biochemistry 43 437–445
A Volbeda MH Charon C Piras EC Hatchikian M Frey JC Fontecillacamps (1995) ArticleTitleCrystal-structure of the nickel–iron hydrogenase from Desulfovibrio gigas Nature 373 580–587
KA Weyer F Lottspeich H Gruenberg F Lang D Oesterhelt H Michel (1987) ArticleTitleAmino acid sequence of the cytochrome subunit of the photosynthetic reaction centre from the purple bacterium Rhodopseudomonas viridis EMBO J 6 2197–2202
TO Yeates H Komiya A Chirino DC Rees JP Allen G Feher (1988) ArticleTitleStructure of the reaction center from Rhodobacter sphaeroides R-26 and 2.4.1: protein-cofactor (bacteriochlorophyll, bacteriopheophytin, and carotenoid). interactions Proc Natl Acad Sci USA 85 7993–7997
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nogi, T., Hirano, Y. & Miki, K. Structural and functional studies on the tetraheme cytochrome subunit and its electron donor proteins: the possible docking mechanisms during the electron transfer reaction. Photosynth Res 85, 87–99 (2005). https://doi.org/10.1007/s11120-004-2416-5
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11120-004-2416-5