Abstract
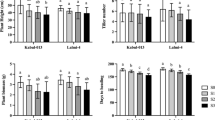
Salinity severely influences growth and grain yield of wheat. Modern breeding efforts have contributed to severe loss of genetic diversity and reduced tolerance to salt stress in cultivated plants. Wild emmer wheat (Triticum dicoccoides), the progenitor of cultivated wheat, is well-adapted to a wide range of environments and exhibits tolerance to abiotic stress. However, there is lack of fundamental knowledge of the mechanism of salt stress tolerance in wild emmer wheat and how it differs from that of the cultivated wheat. By screening wild emmer genotypes, we identified a promising salt-tolerant line from Gitit in the eastern Samaria steppes. We investigated the physiological difference of wild emmer and cultivated wheats in response to salt stress. Our results revealed that salt stress resulted in an increase in lipid peroxidation (malondialdehyde) content and electrolyte leakage, to a greater extent in cultivated wheat genotype, Zheng 9023, than in salt-resistant wild emmer wheat genotype 18-35, but the latter had higher relative dry weight. Differential expression analysis showed that higher transcript induction folds of genes encoding transcription factor were detected in the resistant plants (wild emmer) than in sensitive plants (cultivated wheat) after salt treatment. In conclusion, wild emmer wheat demonstrated better tolerance to salt stress than cultivated wheat, and the higher tolerance of wild emmer wheat is because of high expression of stress-responsive genes encoding transcription factor, including NAC2F, NAC8, DREB3A, MYB3R, and MYB2A. Therefore, our results suggest that wild emmer wheat is an important germplasm for salt tolerance breeding in cultivated wheat.




Similar content being viewed by others
References
Ahmada P, Johnb R, Sarwatc M, Umar S (2008) Responses of proline, lipid peroxidation and antioxidative enzymes in two varieties of Pisum sativum L. under salt stress. Int J Plant Prod 2:353–366
Amor NB, Jiménez A, Megdiche W, Lundqvist M, Sevilla F, Abdelly C (2006) Response of antioxidant systems to NaCl stress in the halophyte Cakile maritima. Physiol Plant 126:446–457
Cai H, Tian S, Liu C, Dong H (2011) Identification of a MYB3R gene involved in drought, salt and cold stress in wheat (Triticum aestivum L.). Gene 485:146–152
Chen L, Ren F, Zhong H, Jiang WM, Li XB (2010) Identification and expression analysis of genes induced by high-salinity and drought stresses in Brassica napus. Acta Biochim Biophys Sin 42:154–164
Chen L, Zhong H, Ren F, Guo QQ, Hu XP, Li XB (2011) A novel cold-regulated gene, COR25, of Brassica napus is involved in plant response and tolerance to cold stress. Plant Cell Rep 30:463–471
Dvorak J, Akhunov ED (2005) Tempos of gene locus deletions and duplications and their relationship to recombination rate during diploid and polyploid evolution in the Aegilops–Triticum alliance. Genetics 171:323–332
Genc Y, McDonald G, Tester M (2007) Reassessment of tissue Na+ concentration as a criterion for salinity tolerance in bread wheat. Plant Cell Environ 30:1486–1498
He Y, Li W, Lv J, Jia Y, Wang M, **a G (2012) Ectopic expression of a wheat MYB transcription factor gene, TaMYB73, improves salinity stress tolerance in Arabidopsis thaliana. J Exp Bot 63:1511–1522
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hernández JA, Almansa MS (2002) Short-term effects of salt stress on antioxidant systems and leaf water relations of leaves. Physiol Plant 115:251–257
Hu L, Li H, Pang H, Fu J (2012) Responses of antioxidant gene, protein and enzymes to salinity stress in two genotypes of perennial ryegrass (Lolium perenne) differing in salt tolerance. J Plant Physiol 169:146–156
Huang S, Sirikhachornkit A, Su X, Faris J, Gill B, Haselkorn R, Gornicki P (2002) Genes encoding plastid acetyl-CoA carboxylase and 3-phosphoglycerate kinase of the Triticum/Aegilops complex and the evolutionary history of polyploid wheat. Proc Natl Acad Sci U S A 99:8133–8138
Koca M, Bor M, Ozdemir F, Turkan I (2007) The effect of salt stress on lipid peroxidation, antioxidative enzymes and proline content of sesame cultivars. Environ Exp Bot 60:344–351
Li XB, Fan XP, Wang XL, Cai L, Yang WC (2005) The cotton ACTIN1 gene is functionally expressed in fibers and participates in fiber elongation. Plant Cell 17:859–875
Liu X, Huang B (2000) Heat stress injury in relation to membrane lipid peroxidation in cree** bentgrass. Crop Sci 40:503–510
Lucas S, Durmaz E, Akpınar BA, Budak H (2011) The drought response displayed by a DRE-binding protein from Triticum dicoccoides. Plant Physiol Bioch 49:346–351
Mao X, Jia D, Li A, Zhang H, Tian S, Zhang X, Jia J, **g R (2011) Transgenic expression of TaMYB2A confers enhanced tolerance to multiple abiotic stresses in Arabidopsis. Funct Integr Genomics 11:445–465
Mao X, Zhang H, Qian X, Li A, Zhao G, **g R (2012) TaNAC2, a NAC-type wheat transcription factor conferring enhanced multiple abiotic stress tolerances in Arabidopsis. J Exp Bot 63:2933–2946
Munns R, Hore RA, James RA, Rebetzke GJ (2000) Genetic variation for improving the salt tolerance of durum wheat. Aust J Agric Res 51:69–74
Nevo E, Chen G (2010) Drought and salt tolerances in wild relatives for wheat and barley improvement. Plant Cell Environ 33:670–685
Nevo E, Krugman T, Beiles A (1993) Genetic resources for salt tolerance in the wild progenitors of wheat (Triticum dicoccoides) and barley (Hordeum spontaneum) in Israel. Plant Breeding 110:338–341
Peleg Z, Fahima T, Abbo S, Krugman T, Nevo E, Yakir D, Saranga Y (2005) Genetic diversity for drought resistance in wild emmer wheat and its ecogeographical associations. Plant Cell Environ 28:176–191
Peleg Z, Saranga Y, Krugman T, Abbo S, Nevo E, Fahima T (2008) Allelic diversity associated with aridity gradient in wild emmer wheat populations. Plant Cell Environ 31:39–49
Peleg Z, Fahima T, Korol AB, Abbo S, Saranga Y (2011) Genetic analysis of wheat domestication and evolution under domestication. J Exp Bot 62:5051–5061
Peng JH, Sun D, Nevo E (2011) Domestication evolution, genetics and genomics in wheat. Mol Breeding 28:281–301
Premchandra GS, Saneoka H, Fujita K, Ogata S (1992) Leaf water relations, osmotic adjustment, cell membrane stability, epi-cuticular wax load and growth as affected by increasing water deficits in sorghum. J Exp Bot 43:1569–1576
Qin F, Shinozaki K, Yamaguchi-Shinozaki K (2011) Achievements and challenges in understanding plant abiotic stress responses and tolerance. Plant Cell Physiol 52:1569–1582
Sekmen AH, Türkan I, Takio S (2007) Differential responses of antioxidative enzymes and lipid peroxidation to salt stress in salt-tolerant Plantago maritima and salt sensitive Plantago media. Physiol Plant 131:399–411
Shavrukov Y, Langridge P, Tester M, Nevo E (2010) Wide genetic diversity of salinity tolerance, sodium exclusion and growth in wild emmer wheat, Triticum dicoccoides. Breeding Sci 60:426–435
Stevens J, Senaratna T, Sivasithamparam K (2006) Salicylic acid induces salinity tolerance in tomato (Lycopersicon esculentum cv. Roma): associated changes in gas exchange, water relations and membrane stabilization. Plant Growth Regul 49:77–83
**a N, Zhang G, Sun YF, Zhu L, Xu LS, Chen XM, Liu B, Yu YT, Wang XJ, Huang LL, Kang ZS (2010) TaNAC8, a novel NAC transcription factor gene in wheat, responds to stripe rust pathogen infection and abiotic stresses. Physiol Mol Plant P 74:394–402
** and transfer for wheat improvement. Euphytica 164:603–614
Zamir D (1995) Improving plant breeding with exotic genetic libraries. Nat Rev Genet 2:983–989
Acknowledgments
This work was supported by the China National Science Foundation grant nos. 31030055 and 31071822 and the Hubei Province National Science Foundation Sciences grant no. ZRY1326.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Liang Chen and **g Ren contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chen, L., Ren, J., Shi, H. et al. Physiological and Molecular Responses to Salt Stress in Wild Emmer and Cultivated Wheat. Plant Mol Biol Rep 31, 1212–1219 (2013). https://doi.org/10.1007/s11105-013-0584-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11105-013-0584-1