Abstract
Aims
In this work, an ammonium-excreting strain (HM053) of A. brasilense was further characterized genetically and biochemically, and its abilities to colonize and promote wheat growth were determined.
Methods
Immunoblot, reverse transcription-qPCR, and DNA sequencing were used for HM053 characterization. To analyze wheat-A. brasilense interaction nifH::gusA fusions in the wild-type FP2 (FP2-7) and HM053 (HM053-36) backgrounds were employed.
Results
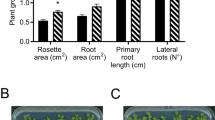
HM053 glutamine synthetase (GS) was not adenylylated in response to an ammonium shock or under any condition tested. Sequencing of the glnA gene revealed a substitution of a proline residue by a leucine at position 347 of the GS. Under axenic growth condition, HM053 was capable of colonizing the surface of wheat roots and increased by 30 and 49% the shoot and root dry weight, respectively, when compared with uninoculated plants, and by 30 and 31% when compared with the parental strain FP2. Although HM053-36 and FP2-7 showed GUS activity located mainly at lateral root emergence points, HM053-36 consistently showed stronger signals and expressed the nifH gene at a level 278 fold higher than strain FP2 in planta, according to qPCR data.
Conclusions
HM053, a spontaneous mutant in GS, increased wheat root and shoot dry weight when compared to the wild-type FP2. HM053 ability to excrete ammonium and fix nitrogen constitutively, even in the presence of high NH4 + concentration, could explain why this mutant has a higher potential to promote plant growth than FP2 and suggests HM053 as a potential nitrogen biofertilizer. However, HM053 should be tested under field conditions to evaluate its abilities to compete with indigenous microflora.




Similar content being viewed by others
Abbreviations
- PGPR:
-
Plant-growth-promoting rhizobacterium (PGPR)
- BNF:
-
Biological nitrogen fixation
- GS:
-
Glutamine synthetase
- GOGAT:
-
Glutamate synthase
- GUS:
-
β-glucuronidase
- CFU:
-
Colony forming units
- D.a.i:
-
Days after inoculation
References
Abell LM, Schineller J, Keck PJ, Villafranca JJ (1995) Effect of metal-ligand mutations on phosphoryl transfer reactions catalyzed by Escherichia coli glutamine synthetase. Biochemistry 34:16695–16702
Alves BJR, Boddey RM, Urquiaga S (2003) The success of BNF in soybean in Brazil. Plant Soil 252:1–9
Araújo LM, Monteiro RA, Souza EM et al (2004) GlnB is specifically required for Azospirillum brasilense NifA activity in Escherichia coli. Res Microbiol 155:491–495. doi:10.1016/j.resmic.2004.03.002
Arcondéguy T, Jack R, Merrick M (2001) PII signal transduction proteins, pivotal players in microbial nitrogen control. Microbiol Mol Biol Rev 65:80–105. doi:10.1128/MMBR.65.1.80-105.2001
Arsene F, Kaminski PA, Elmerich C (1996) Modulation of NifA activity by PII in Azospirillum brasilense: evidence for a regulatory role of the NifA N-terminal domain. J Bacteriol 178:4830–4838
Bottini R, Fulchieri M, Pearce D, Pharis RP (1989) Identification of gibberellins A1, A3, and iso-A3 in cultures of Azospirillum lipoferum. Plant Physiol 90:45–47
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilization: the principle of protein-dye binding. Anal Biochem 72:248–254
Camilios-Neto D, Bonato P, Wassem R et al (2014) Dual RNA-seq transcriptional analysis of wheat roots colonized by Azospirillum brasilense reveals up-regulation of nutrient acquisition and cell cycle genes. BMC Genomics 15:378. doi:10.1186/1471-2164-15-378
Chaney L, Marbach P (1962) Modified reagents of urea and for determination ammonia. Clin Chem 8:130–132
Christiansen-Weniger C, Van Veen J (1991) Nitrogen fixation by Azospirillum brasilense in soil and rhizosphere under controlled environmental conditions. Biol Fertil Soils 12:100–106. doi:10.1007/BF00341483
Cohen AC, Bottini R, Piccoli PN (2007) Azospirillum brasilense Sp 245 produces ABA in chemically-defined culture medium and increases ABA content in arabidopsis plants. Plant Growth Regul 54:97–103. doi:10.1007/s10725-007-9232-9
Curatti L, Rubio LM (2014) Challenges to develop nitrogen-fixing cereals by direct nif-gene transfer. Plant Sci 225:130–137. doi:10.1016/j.plantsci.2014.06.003
de Oliveira Pinheiro R, Boddey LH, James EK et al (2002) Adsorption and anchoring of Azospirillum strains to roots of wheat seedlings. Plant Soil 246:151–166
Dhalla AM, Li BIN, Alibhai ME et al (1994) Regeneration of catalytic activity of glutamine synthetase mutants by chemical activation: exploration of the role of arginines 339 and 359 in activity. Protein Sci 3:476–481
Dobbelaere S, Okon Y (2007) The plant growth-promoting effect and plant responses. In: Elmerich C, Newton WE (eds) Associative and endophytic nitrogen-fixing bacteria and cyanobacterial associations. Springer, Dordrecht, pp. 145–170
Döbereiner J (1992) History and new perspectives of diazotrophs in association with non leguminous plants. Symbiosis 13:1–13
Egener T, Hurek T, Reinhold-Hurek B (1998) Use of green fluorescent protein to detect expression of nif genes of Azoarcus sp. BH72, a grass-associated diazotroph, on rice roots. Mol Plant-Microbe Interact 11:71–75. doi:10.1094/MPMI.1998.11.1.71
Elmerich C, Aubert JP (1971) Synthesis of glutamate by a glutamine: 2-oxo-glutarate amidotransferase (NADP oxidoreductase) in Bacillus megaterium. Biochem Biophys Res Commun 42:371–376
Fadel-Picheth CMT, Souza EM, Rigo LU, Funayama S (1999) Regulation of Azospirillum brasilense nifA gene expression by ammonium and oxygen. FEMS Microbiol Lett 179:281–288
Fallik E, Okon Y (1996) The response of maize (Zea mays) to Azospirillum inoculation in various types of soils in the field. World J Microbiol Biotechnol 12:511–515
Fibach-Paldi S, Burdman S, Okon Y (2012) Key physiological properties contributing to rhizosphere adaptation and plant growth promotion abilities of Azospirillum brasilense. FEMS Microbiol Lett 326:99–108. doi:10.1111/j.1574-6968.2011.02407.x
Fu H, Hartmann A, Lowery RG et al (1989) Posttranslational regulatory system for nitrogenase activity in Azospirillum spp. J Bacteriol 171:4679–4685
García de Salamone IE, Salvo LP, Escobar Ortega JS et al (2010) Field response of rice paddy crop to Azospirillum inoculation: physiology of rhizosphere bacterial communities and the genetic diversity of endophytic bacteria in different parts of the plants. Plant Soil 336:351–362. doi:10.1007/s11104-010-0487-y
Gauthier D, Elmerich C (1977) Relationship between glutamine synthetase and nitrogenase in Spirillum lipoferum. FEMS Microbiol Lett 2:101–104
Gerk LP, Gilchrist K, Kennedy IR (2000) Mutants with enhanced nitrogenase activity in hydroponic Azospirillum brasilense-wheat associations. Appl Environ Microbiol 66:2175–2184. doi:10.1128/AEM.66.5.2175-2184.2000
Hartmann A, Fu H, Burris RH (1986) Regulation of nitrogenase activity by ammonium chloride in Azospirillum spp. J Bacteriol 165:864–870
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Calif Agric. Exp. Stn. Berkeley CA 347:1–32
Huergo L, Souza E, Araujo M et al (2006) ADP-ribosylation of dinitrogenase reductase in Azospirillum brasilense is regulated by AmtB-dependent membrane sequestration of DraG. Mol Microbiol 59:326–337
Huergo LF, Pedrosa FO, Muller-Santos M et al (2012) PII signal transduction proteins: pivotal players in post-translational control of nitrogenase activity. Microbiology 158:176–190. doi:10.1099/mic.0.049783-0
Hungria M, Campo RJ, Souza EM, Pedrosa FO (2010) Inoculation with selected strains of Azospirillum brasilense and A. lipoferum improves yields of maize and wheat in Brazil. Plant Soil 331:413–425. doi:10.1007/s11104-009-0262-0
Hurek T, Reinhold-hurek B, Montagu MVAN, Kellenberger E (1994) Root colonization and systemic spreading of Azoarcus strain BH72 in grasses. J Bacteriol 176:1913–1923
James EK (2000) Nitrogen fixation in endophytic and associative symbiosis. Field Crops Res 65:197–209
Karnovsky M (1965) A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. J Cell Biol 27:137A–138A
Koutroubas S, Papakosta D, Gagianas A (1998) The importance of early dry matter and nitrogen accumulation in soybean yield. Eur J Agron 9:1–10
Kupferschmied P, Maurhofer M, Keel C (2013) Promise for plant pest control: root-associated pseudomonads with insecticidal activities. Fron Plant Sci 4:287. doi:10.3389/fpls.2013.00287
Latorre C, Lee JH, Spiller H, Shanmugam KT (1986) Ammonium ion-excreting cyanobacterial mutant as a source of nitrogen for growth of rice: a feasibility study. Biotechnol Lett 8:507–512. doi:10.1007/BF01025211
Liaw SH, Eisenberg D (1994) Structural model for the reaction mechanism of glutamine synthetase, based on five crystal structures of enzyme-substrate complexes. Biochemistry 33:675–681
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408. doi:10.1006/meth.2001.1262
Machado, Funayama S, Rigo LU, Pedrosa F (1991) Excretion of ammonium by Azospirillum brasilense mutants resistant to ethylenediamine. Can J Microbiol 37:549–553
Martínez-Morales L, Soto-Urzúa L, Baca B, Sánchez-Ahédo J (2003) Indole-3-butyric acid (IBA) production in culture medium by wild strain Azospirillum brasilense. Media FEMS Microbiol Lett 228:167–173. doi:10.1016/S0378-1097(03)00694-3
Meers J, Tempest D (1970) “Glutamine(amide): 2-oxoglutarate amino transferase oxido-reductase (NADP)”, an enzyme involved in the synthesis of glutamate by some bacteria. J Gen Microbiol 64:187–194
Mendes I, Hungria M, Vargas M (2003) Soybean response to starter nitrogen and Bradyrhizobium inoculation on Cerrado oxisol under no-tillage and conventional tillage systems. Rev Bras Cienc Solo 27:81–87
Moure VR, Danyal K, Yang Z-Y et al (2013) The nitrogenase regulatory enzyme dinitrogenase reductase ADP-ribosyltransferase (DraT) is activated by direct interaction with the signal transduction protein GlnB. J Bacteriol 195:279–286. doi:10.1128/JB.01517-12
Okon Y, Vanderleyden J (1997) Root associatied Azospirillum species can stimulate plants. ASM News 63:364–370
Ortiz-Marquez JCF, Do Nascimento M, delos Dublan MA, Curatti L (2012) Association with an ammonium-excreting bacterium allows diazotrophic culture of oil-rich eukaryotic microalgae. Appl Environ Microbiol 78:2345–2352. doi:10.1128/AEM.06260-11
Pankievicz VCS, Do Amaral FP, Santos KFDN et al (2015) Robust biological nitrogen fixation in a model grass-bacterial association. Plant J 81:907–919. doi:10.1111/tpj.12777
Pedrosa FO, Yates MG (1984) Regulation of nitrogen fixation (nif) genes of Azospirillum brasilense by nifA and ntrC (glnG) type genes. FEMS Microbiol Lett 55:95–101
Ryu C, Farag MA, Hu C et al (2004) Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol 134:1017–1026. doi:10.1104/pp.103.026583
Schloter M, Hartmann A (1998) Endophytic and surface colonization of wheat roots (Triticum aestivum) by different Azospirillum brasilense strains studied with strain-specific monoclonal antibodies. Symbiosis 25:159–179
Sij JW, Turner FT, Craigmiles JP (1979) “Starter nitrogen”; fertilization in soybean culture. Commun Soil Sci Plant Anal 10:1451–1457. doi:10.1080/00103627909366999
Spaepen S, Dobbelaere S, Croonenborghs A, Vanderleyden J (2008) Effects of Azospirillum brasilense indole-3-acetic acid production on inoculated wheat plants. Plant Soil 312:15–23. doi:10.1007/s11104-008-9560-1
Spiller H, Gunasekaran M (1990) Ammonia-excreting mutant strain of the cyanobacterium Anabaena variabilis supports growth of wheat. Appl Microbiol Biotechnol 33:477–480. doi:10.1007/BF00176670
Srivastava A, Tripathi AK (2006) Adenosine diphosphate ribosylation of dinitrogenase reductase and adenylylation of glutamine synthetase control ammonia excretion in ethylenediamine-resistant mutants of Azospirillum brasilense Sp7. Curr Microbiol 53:317–323. doi:10.1007/s00284-006-0058-x
Steenhoudt O, Vanderleyden J (2000) Azospirillum, a free-living nitrogen-fixing bacterium closely associated with grasses: genetic, biochemical and ecological aspects. FEMS Microbiol Rev 24:487–506
Tien TM, Gaskins MH, Hubbell DH (1979) Plant growth substances produced by Azospirillum brasilense and their effect on the growth of pearl millet (Pennisetum americanum L.) Appl. Environ Microbiol 37:1016–1024
Van Dommelen A, Keijers V, Wollebrants A, Vanderleyden J (2003) Phenotypic changes resulting from distinct point mutations in the Azospirillum brasilense glnA Gene, encoding glutamine synthetase. Appl Environ Microbiol 7028:5699–5701. doi:10.1128/AEM.69.9.5699-5701.2003
Van Dommelen A, Croonenborghs A, Spaepen S, Vanderleyden J (2009) Wheat growth promotion through inoculation with an ammonium-excreting mutant of Azospirillum brasilense. Biol Fertil Soils 45:549–553. doi:10.1007/s00374-009-0357-z
Vessey JK (2003) Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 255:571–586. doi:10.1023/A:1026037216893
Vitorino JC, Steffens MB, Machado HB et al (2001) Potential roles for the glnB and ntrYX genes in Azospirillum brasilense. FEMS Microbiol Lett 201:199–204. doi:10.1111/j.1574-6968.2001.tb10757.x
Witmer MR, Palmieri-Young D, Villafranca JJ (1994) Probing the catalytic roles of n2-site glutamate residues in Escherichia coli glutamine synthetase by mutagenesis. Protein Sci 3:1746–1759
Wood CC, Islam N, Ritchie RJ, Kennedy IR (2001) A simplified model for assessing critical parameters during associative 15N2 fixation between Azospirillum and wheat. Aust J Plant Physiol 28:969–974. doi:10.1071/PP01036
Acknowledgements
This work was supported by the National Institute of Science and Technology on Biological Nitrogen Fixation (INCT/CNPq). We thank Roseli Prado, Valter A. Baura, and Marilza Doroty Lamour for the technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Euan K. James.
Rights and permissions
About this article
Cite this article
Santos, K.F.D.N., Moure, V.R., Hauer, V. et al. Wheat colonization by an Azospirillum brasilense ammonium-excreting strain reveals upregulation of nitrogenase and superior plant growth promotion. Plant Soil 415, 245–255 (2017). https://doi.org/10.1007/s11104-016-3140-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-016-3140-6