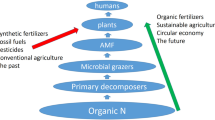
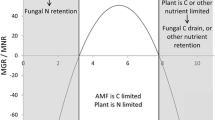
Abstract
Background
Arbuscular mycorrhizal fungi (AMF) form mutualistic symbioses with c. two-thirds of all land plants. Traditionally, it was thought that they played no role in nitrogen (N) acquisition for their host, despite early evidence to the contrary. More recently, this perception has changed radically, with the demonstration that AMF can acquire N from both inorganic and organic N sources and transfer some of this N to their host plant.
Scope
This review discusses the current evidence for AMF N uptake, transport and plant transfer under different experimental conditions and highlights key questions that remain to be resolved. The relevance of this AMF N acquisition pathway is discussed both in relation to host plant and fungal N nutrition. The importance of interactions with the soil community and subsequent implications for soil N cycling are also highlighted.
Conclusions
Reported AMF contribution to plant N varies widely, but the reasons for this variability are unclear. In low N systems even small amounts of ‘extra’ N may confer the plant with a competitive advantage, but it is also likely that competition for N between symbionts occurs. To advance this area, a more mechanistic approach is required that treats the fungus as a Darwinian organism rather than a mere extension of the plant. Application of genomics and metabolomics technologies to this topic should enable resolution of some of the key questions outlined in this review.



Similar content being viewed by others
References
Ames RN, Reid CPP, Porter LK, Cambardella C (1983) Hyphal uptake and transport of nitrogen from two 15N-labelled sources by Glomus mosseae, a vesicular-arbuscular mycorrhizal fungus. New Phytol 95:381–396
Amora-Lazcano E, Vazquez MM, Azcón R (1998) Response of nitrogen-transforming microorganisms to arbuscular mycorrhizal fungi. Biol Fertil Soils 27:65–70
Antoninka A, Reich PB, Johnson NC (2011) Seven years of carbon dioxide enrichment, nitrogen fertilization and plant diversity influence arbuscular mycorrhizal fungi in a grassland ecosystem. New Phytol 192:200–214
Asghari HR, Cavagnaro TR (2011) Arbuscular mycorrhizas enhance plant interception of leached nutrients. Funct Plant Biol 38:219–226
Atkin OK, Sherlock D, Fitter AH, Jarvis S, Hughes JK, Campbell C, Hurry V, Hodge A (2009) Temperature dependence of respiration in roots colonized by arbuscular mycorrhizal fungi. New Phytol 182:188–199
Atul-Nayyar A, Hamel C, Hanson K, Germida J (2009) The arbuscular mycorrhizal symbiosis links N mineralization to plant demand. Mycorrhiza 19:239–246
Azcón R, Rodríguez R, Amora-Lazcano E, Ambrosano E (2008) Uptake and metabolism of nitrate in mycorrhizal plants as affected by water availability and N concentration in soil. Eur J Soil Sci 59:131–138
Baas R, van der Warf A, Lambers H (1989) Root respiration and growth in Plantago major as affected by vesicular-arbuscular mycorrhizal infection. Plant Physiol 91:227–232
Bago B, Azcón-Agullar C (1997) Changes in the rhizosphere pH induced by arbuscular mycorrhizal formation in onion (Allium cepa L.). Z Pflanzenernähr Bodenkd 160:333–339
Bago B, Vierheilig H, Piché Y, Azcón-Agullar C (1996) Nitrate depletion and pH changes induced by the extraradical mycelium of the arbuscular mycorrhizal fungus Glomus intraradices grown in monoxenic culture. New Phytol 133:273–280
Barrett G, Campbell CD, Fitter AH, Hodge A (2011) The arbuscular mycorrhizal fungus Glomus hoi can capture and transfer nitrogen from organic patches to its associated host plant at low temperature. Appl Soil Ecol 48:102–105
Bender SF, Plantenga F, Neftel A, Jocher M, Oberholzer H-R, Köhl L, Giles M, Daniell TJ, van der Heijden MGA (2013) Symbiotic relationships between soil fungi and plants reduce N2O emissions from soil. ISME J Online Early. doi:10.1038/ismej.2013.224
Blanke V, Wagner M, Renker C, Lippert H, Michulitz M, Kuhn AJ, Buscot F (2011) Arbuscular mycorrhizas in phosphate-polluted soil: interrelations between root colonization and nitrogen. Plant Soil 343:379–392
Booth MS, Stark JM, Rastetter E (2005) Controls on nitrogen cycling in terrestrial ecosystems: A synthetic analysis of literature data. Ecol Monogr 75:139–157
Bryla DR, Eissenstat DM (2005) Respiratory costs of mycorrhizal associations. In: Lambers H, Ribas Carbo M (eds) Plant Respiration. Springer, The Netherlands, pp 207–224
Cahill JF, McNickle GG (2011) The behavioural ecology of nutrient foraging by plants. Annu Rev Ecol Evol Syst 42:289–311
Canfield DE, Glazer AN, Falkowski PG (2010) The evolution and future of Earth’s nitrogen cycle. Science 330:192–196
Cappellazzo G, Lanfranco L, Bonfante P (2007) A limiting source of organic nitrogen induces specific transcriptional responses in the extraradical structures of the endomycorrhizal fungus Glomus intraradices. Curr Genet 51:59–70
Cappellazzo G, Lanfranco L, Fitz M, Wipf D, Bonfante P (2008) Characterization of an amino acid permease from the endomycorrhizal fungus Glomus mosseae. Plant Physiol 147:429–437
Cavagnaro TR, Jackson LE, Scow KM, Hristova KR (2007) Effects of arbuscular mycorrhizas on ammonia oxidizing bacteria in an organic farm soil. Microb Ecol 54:618–626
Cavagnaro T, Barrios-Masias F, Jackson L (2012) Arbuscular mycorrhizas and their role in plant growth, nitrogen interception and soil gas efflux in an organic production system. Plant Soil 353:1–14
Chen SK, Edwards CA, Subler S (2001) Effects of the fungicides benomyl, captan and chlorothalonil on soil microbial activity and nitrogen dynamics in laboratory incubations. Soil Biol Biochem 33:1971–1980
Cheng L, Booker FL, Tu C, Burkey KO, Zhou L, Shew HD, Rufty TW, Hu S (2012) Arbuscular mycorrhizal fungi increase organic carbon decomposition under elevated CO2. Science 337:1084–1087
Cheng W, Parton WJ, Gonzalez-Meler MA, Phillips R, Asao S, McNickle GG, Brzostek E, Jastrow JD (2014) Synthesis and modelling perspectives of rhizosphere priming. New Phytol 201:31–44
Chiariello N, Hickman JC, Mooney HA (1982) Endomycorrhizal role for interspecific transfer of phosphorus in a community of annual plants. Science 217:941–943
Christensen S, Griffiths BS, Ekelund F, Rønn R (1992) Huge increase in bacterivores on freshly killed barley roots. FEMS Microbiol Ecol 86:303–310
Clarholm M (1985) Interactions of bacteria, protozoa and plants leading to mineralization of soil nitrogen. Soil Biol Biochem 17:181–187
Croft SA, Hodge A, Pitchford JW (2012) Optimal root proliferation strategies: the roles of nutrient heterogeneity, competition and mycorrhizal networks. Plant Soil 351:191–206
Cruz C, Esgâârd H, Trujillo C, Ambus P, Requena N, Martins-Loução MA, Jakobsen I (2007) Enzymatic evidence for the key role of arginine in nitrogen translocation by arbuscular mycorrhizal fungi. Plant Physiol 144:782–792
Cui M, Caldwell MM (1996a) Facilitation of plant phosphate acquisition by arbuscular mycorrhizas from enriched soil patches. I. Roots and hyphae exploiting the same soil volume. New Phytol 133:453–460
Cui M, Caldwell MM (1996b) Facilitation of plant phosphate acquisition by arbuscular mycorrhizas from enriched soil patches. II. Hyphae exploiting root-free soil. New Phytol 133:461–467
De Nobili M, Contin M, Mondini C, Brookes PC (2001) Soil microbial biomass is triggered into activity by trace amounts of substrate. Soil Biol Biochem 33:1163–1170
Drew MC (1975) Comparison of the effects of a localized supply of phosphate, nitrate, ammonium and potassium on the growth of the seminal root system, and the shoot, in barley. New Phytol 75:479–490
Estaún V, Calvet C, Hayman DS (1987) Influence of plant genotype on mycorrhizal infection: response of three pea cultuivars. Plant Soil 103:295–298
Fellbaum CR, Gachomo EW, Beesetty Y, Choudhari S, Strahan GD, Pfeffer PE, Kiers ET, Bücking H (2012) Carbon availability triggers fungal nitrogen uptake and transport in arbuscular mycorrhizal symbiosis. Proc Natl Acad Sci U S A 109:2666–2671
Garcia MO, Ovasapyan T, Greas M, Treseder KK (2008) Mycorrhizal dynamics under elevated CO2 and nitrogen fertilization in a warm temperate forest. Plant Soil 303:301–310
Geisseler D, Horwath WR, Joergensen RG, Ludwig B (2010) Pathways of nitrogen utilization by soil microorganisms – a review. Soil Biol Biochem 42:2058–2067
Gomez SK, Javot H, Deewatthanawong P, Torres-Jerez I, Tnag Y, Blancaflor EB, Udvardi MK, Harrison MJ (2009) Medicago truncatula and Glomus intraradices gene expression in cortical cells harbouring arbuscules in the arbuscular mycorrhizal symbiosis. BMC Plant Biol 9:10. doi:10.1186/1471-2229-9-10
Govindarajulu M, Pfeffer PE, ** H, Abubaker J, Douds DD, Allen JW, Bücking H, Lammers PJ, Shachar-Hill Y (2005) Nitrogen transfer in the arbuscular mycorrhizal symbiosis. Nature 435:819–823
Gryndler M, Jansa J, Hršelová H, Chvátalová I, Vosátka M (2003) Chitin stimulates development and sporulation of arbuscular mycorrhizal fungi. Appl Soil Ecol 22:283–287
Guether M, Neuhäuser B, Balestrini R, Dynowski M, Ludewig U, Bonfante P (2009a) A mycorrhizal-specific ammonium transporter from Lotus japonicus acquires nitrogen released by arbuscular mycorrhizal fungi. Plant Physiol 150:73–83
Guether M, Balestrini R, Hannah M, Me J, Udvardi MK, Bonfante P (2009b) Genome-wide reprogramming of regulatory networks, transport, cell wall and membrane biogenesis during arbuscular mycorrhizal symbiosis in Lotus japonicus. New Phytol 182:200–212
Guether M, Volpe V, Balestrini R, Requena N, Wipf D, Bonfante P (2011) LjLHT1.2—a mycorrhiza-inducible plant amino acid transporter from Lotus japonicus. Biol Fertil Soils 47:925–936
Harley JL (1989) The significance of mycorrhizal. Mycol Res 92:129–139
Hawkins HJ, George E (2001) Reduced 15N-nitrogen transport through arbuscular mycorrhizal hyphae to Triticum aestivum L. supplied with ammonium vs. nitrate nutrition. Ann Bot 87:303–311
Hawkins HJ, Johansen A, George E (2000) Uptake and transport of organic and inorganic nitrogen by arbuscular mycorrhizal fungi. Plant Soil 226:275–285
He XH, Minggang X, Qiu GY, Zhou J (2009) Use of 15N stable isotope to quantify nitrogen transfer between mycorrhizal plants. J Plant Ecol UK 2:107–118
Hepper CM, Warner A (1983) Role of organic matter in growth of a vesicular-arbuscular mycorrhizal fungus in soil. Trans Br Mycol Soc 81:155–156
Herman D, Firestone M, Nuccio E, Hodge A (2012) Interactions between an arbuscular mycorrhizal fungus and a soil microbial community mediating litter decomposition. FEMS Microbiol Ecol 80:236–247
Hill PW, Quilliam RS, DeLuca TH, Farrar J, Farrell M, Roberts P, Newsham KK, Hopkins DW, Bardgett RD, Jones DL (2011) Acquisition and assimilation of nitrogen as peptide-bound and D-enantiomers of amino acids by wheat. PLoS ONE 6:e19220
Hino T, Matsumoto Y, Nagano S, Sugimoto H, Fukumori Y, Murata T, Iwata S, Shiro Y (2010) Structural basis of biological N2O generation by bacterial nitric oxide reductase. Science 330:1666–1670
Hodge A (1996) Impact of elevated CO2 on mycorrhizal associations and implications for plant growth. Biol Fertil Soils 23:388–398
Hodge A (2000) Microbial ecology of the arbuscular mycorrhiza. FEMS Microb Ecol 32:91–96
Hodge A (2001) Arbuscular mycorrhizal fungi influence decomposition of, but not plant nutrient capture from, glycine patches in soil. New Phytol 151:725–734
Hodge A (2003a) Plant nitrogen capture from organic matter as affected by spatial dispersion, interspecific competition and mycorrhizal colonisation. New Phytol 157:303–314
Hodge A (2003b) N capture by Plantago lanceolata and Brassica napus from organic material: the influence of spatial dispersion, plant competition and an arbuscular mycorrhizal fungus. J Exp Bot 54:2331–2342
Hodge A (2004) The plastic plant: root responses to heterogeneous supplies of nutrients. New Phytol 162:9–24
Hodge A (2006) Plastic plants and patchy soils. J Exp Bot 57:401–411
Hodge A, Fitter AH (2010) Substantial nitrogen acquisition by arbuscular mycorrhizal fungi from organic material has implications for N cycling. Proc Natl Acad Sci USA 107:13754–13759
Hodge A, Fitter AH (2013) Microbial mediation of plant competition and community structure. Funct Ecol 27:865–875
Hodge A, Millard P (1998) Effect of elevated CO2 on carbon partitioning and exudate release from Plantago lanceolata seedlings. Physiol Plant 103:280–286
Hodge A, Alexander IJ, Gooday GW (1995) Chitinolytic enzymes of pathogenic and ectomycorrhizal fungi. Mycol Res 99:935–941
Hodge A, Stewart J, Robinson D, Griffiths BS, Fitter AH (1998) Root proliferation, soil fauna and plant nitrogen capture from nutrient-rich patches in soil. New Phytol 139:479–494
Hodge A, Robinson D, Griffiths BS, Fitter AH (1999a) Why plants bother: root proliferation results in increased nitrogen capture from an organic patch when two grasses compete. Plant Cell Environ 22:811–820
Hodge A, Stewart J, Robinson D, Griffiths BS, Fitter AH (1999b) Plant, soil fauna and microbial responses to N-rich organic patches of contrasting temporal availability. Soil Biol Biochem 31:1517–1530
Hodge A, Robinson D, Griffiths BS, Fitter AH (1999c) Nitrogen capture by plants grown in N-rich organic patches of contrasting size and strength. J Exp Bot 50:1243–1252
Hodge A, Robinson D, Fitter A (2000a) Are microorganisms more effective than plants at competing for nitrogen? Trends Plant Sci 5:304–308
Hodge A, Stewart J, Robinson D, Griffiths BS, Fitter AH (2000b) Spatial and physical heterogeneity of N supply from soil does not influence N capture by two grass species. Funct Ecol 14:645–653
Hodge A, Robinson D, Fitter AH (2000c) An arbuscular mycorrhizal inoculum enhances root proliferation in, but not nitrogen capture from, nutrient-rich patches in soil. New Phytol 145:575–584
Hodge A, Stewart J, Robinson D, Griffiths BS, Fitter AH (2000d) Plant N capture and microfaunal dynamics from decomposing grass and earthworm residues in soil. Soil Biol Biochem 32:1763–1772
Hodge A, Campbell CD, Fitter AH (2001) An arbuscular mycorrhizal fungus accelerates decomposition and acquires nitrogen directly from organic material. Nature 413:297–299
Hoyle FC, Murphy DV, Brookes PC (2008) Microbial response to the addition of glucose in low-fertility soils. Biol Fertil Soils 44:571–579
Hughes JK, Hodge A, Fitter AH, Atkin OK (2008) Mycorrhizal respiration: implications for global scaling relationships. Trends Plant Sci 13:583–588
Jalonen R, Nygren P, Jorge S (2009) Transfer of nitrogen from a tropical legume tree to an associated fodder grass via root exudation and common mycelia networks. Plant Cell Environ 32:1366–1376
** H, Pfeffer PE, Douds DD, Piotrowski E, Lammers PJ, Shachar-Hill Y (2005) The uptake, metabolism, transport and transfer of nitrogen in an arbuscular mycorrhizal symbiosis. New Phytol 168:687–696
Johansen A, Jakobsen I, Jensen ES (1992) Hyphal transport of 15N-labelled nitrogen by a vesicular-arbuscular mycorrhizal fungus and its depletion of inorganic soil N. New Phytol 122:281–288
Johansen A, Finlay RD, Olsson PA (1996) Nitrogen metabolism of external hyphae of the arbuscular mycorrhizal fungus Glomus intraradices. New Phytol 133:705–712
Johnson D, Leake JR, Ostle N, Ineson P, Read DJ (2002) In situ 13CO2 pulse-labelling of upland grassland demonstrates a rapid pathway of carbon flux from arbuscular mycorrhizal mycelia to the soil. New Phytol 153:327–334
Johnson NC, Rowland DL, Corkidi L, Egerton-Warburton LM, Allen EB (2003) Nitrogen enrichment alters mycorrhizal allocation at five mesic to semiarid grasslands. Ecology 84:1895–1908
Johnson NC, Wilson GWT, Bowker MA, Wilson JA, Miller RM (2010) Resource limitation is a driver of local adaptation in mycorrhizal symbioses. Proc Natl Acad Sci USA 107:2093–2098
Jones DL, Hodge A, Kuzyakov Y (2004) Plant and mycorrhizal regulation of rhizodeposition. New Phytol 163:459–480
Jones DL, Healey JR, Willet VB, Farrar JF, Hodge A (2005) Dissolved organic nitrogen uptake by plants—an important N uptake pathway? Soil Biol Biochem 37:413–423
Jumpponen A, Trowbridge J, Mandyam K, Johnson L (2005) Nitrogen enrichment causes minimal changes in arbuscular mycorrhizal colonization but shifts community composition-evidence from rDNA data. Biol Fertil Soils 41:217–224
Kähkölä A-K, Nygren P, Leblanc HA, Pennanen T, Pietikäinen J (2012) Leaf and root litter of a legume tree as nitrogen sources for cacaos with different root colonisation by arbuscular mycorrhizae. Nutr Cycl Agroecosyst 92:51–65
Karanika ED, Mamolos AP, Alifragis DA, Kalburtji KL, Veresoglou DS (2008) Arbuscular mycorrhizas contribution to nutrition, productivity, structure and diversity of plant community in mountainous herbaceous grassland of northern Greece. Plant Ecol 199:225–234
Karasawa T, Hodge A, Fitter AH (2012) Growth, respiration and nutrient acquisition by the arbuscular mycorrhizal fungus Glomus mosseae and its host plant Plantago lanceolata in cooled soil. Plant Cell Environ 35:819–828
Kim K, Yim W, Trivedi P, Madhaiyan M, Boruah HPD, Islam MR, Lee G, Sa T (2010) Synergistic effects of inoculating arbuscular mycorrhizal fungi and Methylobacterium oryzae strains on growth and nutrient uptake of red pepper (Capsicum annuum L.). Plant Soil 327:429–440
Klopatek CC, Klopatek JM (1997) Nitrifiers and mycorrhizae in pristine and grazed Pinyon-Juniper ecosystems. Arid Soil Res Rehabil 11:331–332
Koller R, Scheu S, Bonkowski M, Robin C (2013a) Protozoa stimulate N uptake and growth of arbuscular mycorrhizal plants. Soil Biol Biochem 65:204–210
Koller R, Rodriguez A, Robin C, Scheu S, Bonkowski M (2013b) Protozoa enhance foraging efficiency of arbuscular mycorrhizal fungi for mineral nitrogen from organic matter in soil to the benefit of host plants. New Phytol 199:203–211
Krüger M, Krüger C, Walker C, Stockinger H, Schußler A (2012) Phylogenetic reference data for systematics and phylotaxonomy of arbuscular mycorrhizal fungi from phylum to species level. New Phytol 193:970–984
Leigh J, Hodge A, Fitter AH (2009) Arbuscular mycorrhizal fungi can transfer substantial amounts of nitrogen to their host plant from organic material. New Phytol 181:199–207
Leigh J, Fitter AH, Hodge A (2011) Growth and symbiotic effectiveness of an arbuscular mycorrhizal fungus in organic matter in competition with soil bacteria. FEMS Microbiol Ecol 76:428–438
Leininger S, Urich T, Schloter M, Schwark L, Qi J, Nicol GW, Prosser JI, Schuster SC, Schleper C (2006) Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 442:806–809
Li X-L, George E, Marschner H (1991) Phosphorus depletion and pH decrease at the root-soil and hyphae-soil interfaces of VA mycorrhizal white clover fertilized with ammonium. New Phytol 119:397–404
López-Pedrosa A, González-Guerrero M, Valderas A, Acón-Aguilar C, Ferrol N (2006) GintAMT1 encodes a functional high-affinity ammonium transporter that is expressed in the extraradical mycelium of Glomus intraradices. Fungal Genet Biol 43:102–110
Mäder P, Vierheilig H, Streitwolf-Engel R, Boller T, Frey B, Christine P, Wiemken A (2000) Transport of 15N from a soil compartment separated by a polytetrafluoroethylene membrane to plant roots via the hyphae of arbuscular mycorrhizal fungi. New Phytol 146:155–161
Marschner H, Dell B (1994) Nutrient uptake in mycorrhizal symbiosis. Plant Soil 159:89–102
Marschner H, Römheld V, Horst WJ, Martin P (1986) Root-induced changes in the rhizosphere: importance for the mineral nutrition of plants. Z Pflanzenernähr Bodenkd 149:441–456
McAllister CB, Garciaromera I, Martin J, Godeas A, Ocampo JA (1995) Interaction between Aspergillus niger van Tiegh and Glomus mosseae (Nicol and Gerd) Gerd and Trappe. New Phytol 129:309–316
Medina A, Jakobsen I, Egsgaard H (2011) Sugar beet waste and its component ferulic acid inhibits external mycelium of arbuscular mycorrhizal fungus. Soil Biol Biochem 43:1456–1463
Mikkelsen BL, Rosendahl S, Jakobsen I (2008) Underground resource allocation between individual networks of mycorrhizal fungi. New Phytol 180:890–898
Mosse B (1959) Observations on the extrametrical mycelium of a vesicular-arbuscular endophyte. Trans Br Mycol Soc 42:439–448
Näsholm T, Ekblad A, Nordin A, Giesler R, Högberg M, Högberg P (1998) Boreal forest plants take up organic nitrogen. Nature 392:914–916
Nazeri NK, Lambers H, Tibbett M, Ryan MH (2014) Moderating mycorrhizas: arbuscular mycorrhizas modify rhizosphere chemistry and maintain plant phosphorus status within narrow boundaries. Plant Cell Environ 37:911–921
Newsham KK, Fitter AH, Watkinson AR (1995) Multi-functionality and biodiversity in arbuscular mycorrhizas. Trends Ecol Evol 10:407–411
Nicol GW, Prosser JI (2011) Strategies to determine diversity, growth and activity of ammonia-oxidisign archaea in soil. Methods Enzymol 496:3–34
Nicolson TH (1959) Mycorrhiza in the Gramineae: I. Vesicular-arbuscular endophytes, with special reference to the external phase. Trans Br Mycol Soc 42:421–438
Nordin A, Högberg P, Näsholm T (2001) Soil nitrogen form and plant nitrogen uptake along a boreal forest productivity gradient. Oecologia 129:125–132
Nuccio EE, Hodge A, Pett-Ridge J, Herman DJ, Weber PK, Firestone MK (2013) An arbuscular mycorrhizal fungus significantly modifies the soil bacterial community and nitrogen cycling during litter decomposition. Environ Microbiol 15:1870–1881
Olsson PA, Burleigh SH, van Aarle IM (2005) The influence of external nitrogen on carbon allocation to Glomus intraradices in monoxenic arbuscular mycorrhiza. New Phytol 168:677–686
Pearson JN, Jakobsen I (1993) The relative contribution of hyphae and roots to phosphorus uptake by arbuscular mycorrhizal plants, measured by dual labelling with 32P and 33P. New Phytol 124:489–494
Quilliam RS, Hodge A, Jones DL (2010) Sporulation of arbuscular mycorrhizal fungi in organic-rich patches following host excision. Appl Soil Ecol 46:247–250
Rains KC, Bledsoe CS (2007) Rapid uptake of the 15N -ammonium and glycine-13C, 15N by arbuscular and ericoid mycorrhizal plants native to a Northern California coastal pygmy forest. Soil Biol Biochem 39:1078–1086
Rasmussen J, Sauheitl L, Eriksen J, Kuzyakov Y (2010) Plant uptake of dual-labeled organic N biased by inorganic C uptake: results of a triple labeling study. Soil Biol Biochem 42:524–527
Ravnskov S, Larsen J, Olsson PA, Jakobsen I (1999) Effects of various organic compounds on growth and phosphorus uptake of an arbuscular mycorrhizal fungus. New Phytol 141:517–524
Read DJ (1991) Mycorrhizas in ecosystems. Experientia 47:376–391
Read D, Perez-Moreno J (2003) Mycorrhizas and nutrient cycling in ecosystems—a journey towards relevance? New Phytol 157:475–492
Redecker D, Schußler A, Stockinger H, Stürmer SL, Morton JB, Walker C (2013) An evidence-based consensus for the classification of arbuscular mycorrhizal fungi (Glomeromycota). Mycorrhiza 23:515–531
Remy W, Taylor TN, Haas H, Kerp H (1994) Four-hundred-million-year-old vesicular-arbuscular mycorrhizae. Proc Natl Acad Sci U S A 91:11841–11843
Reynolds HL, Hartley AE, Vogelsang KM, Bever JD, Schultz PA (2005) Arbuscular mycorrhizal fungi do not enhance nitrogen acquisition and growth of old-field perennials under low nitrogen supply in glasshouse culture. New Phytol 167:869–880
Robinson D, Hodge A, Griffiths BS, Fitter AH (1999) Plant root proliferation in nitrogen-rich patches confers competitive advantage. Proc R Soc Lond B Biol Sci 266:431–435
Rooney DC, Prosser JI, Bending GD, Baggs EM, Killham K, Hodge A (2011) Effect of arbuscular mycorrhizal colonisation on the growth and phosphorus nutrition of Populus euramericana c.v. Ghoy. Biomass Bioenergy 35:4605–4612
Sanders FE, Tinker PB (1973) Phosphate flow into mycorrhizal roots. Pestic Sci 4:385–395
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Academic, London
Smith SE, Smith FA (2011) Roles of arbuscular mycorrhizas in plant nutrition and growth: new paradigms from cellular to ecosystem scales. Annu Rev Plant Biol 62:227–250
Smith FA, Grace EJ, Smith SE (2009) More than a carbon economy: nutrient trade and ecological sustainability in facultative arbuscular mycorrhizal symbiosis. New Phytol 182:347–358
St John TV, Coleman DC, Reid CPP (1983) Association of vesicular-arbuscular mycorrhizal hyphae with soil organic particles. Ecology 64:957–959
Staddon PL, Ramsey CB, Ostle N, Ineson P, Fitter AH (2003) Rapid turnover of hyphae of mycorrhizal fungi determined by AMS microanalysis of 14C. Science 300:1138–1140
Stockinger H, Walker C, Schußler A (2009) ‘Glomus intraradices DAOM197198’, a model fungus in arbuscular mycorrhiza research, is not Glomus intraradices. New Phytol 183:1176–1187
Tanaka Y, Yano K (2005) Nitrogen delivery to maize via mycorrhizal hyphae depends on the form of N supplied. Plant Cell Environ 28:1247–1254
Tobar R, Azcón R, Barea JM (1994) Improved nitrogen uptake and transport from 15N-labelled nitrate by external hyphae of arbuscular mycorrhizal under water-stressed conditions. New Phytol 126:119–122
Toljander JF, Lindahl BD, Paul LR, Elfstrand M, Finlay RD (2007) Influence of arbuscular mycorrhizal mycelial exudates on soil bacterial growth and community structure. FEMS Microbiol Ecol 61:295–304
Toussaint J-P, St-Arnoud M, Charest C (2004) Nitrogen transfer and assimilation between the arbuscular mycorrhizal fungus Glomus intraradices Schenk & Smith and Ri T-DNA roots of Daucus carota L. in an in vitro compartment system. Can J Microbiol 50:251–260
Treseder KK (2004) A meta-analysis of mycorrhizal responses to nitrogen, phosphorus, and atmospheric CO2 in field studies. New Phytol 164:347–355
Treseder KK (2013) The extent of mycorrhizal colonisation of roots and its influence on plant growth and phosphorus content. Plant Soil 371:1–13
Treseder KK, Allen MF (2002) Direct nitrogen and phosphorus limitation of arbuscular mycorrhizal fungi: a model and field test. New Phytol 155:507–515
Tu C, Booker FL, Watson DM, Chen X, Rufty TW, Shi W, Hu S (2006) Mycorrhizal mediation of plant N acquisition and residue decomposition: impact of mineral N inputs. Glob Chang Biol 12:793–803
van der Heijden MGA, Streitwolf-Engel R, Riedl R, Siegrist S, Neudecker A, Ineichen K, Boller T, Wiemken A, Sanders IR (2006) The mycorrhizal contribution to plant productivity, plant nutrition and soil structure in experimental grassland. New Phytol 172:739–752
van der Heijden MGA, Bardgett RD, van Straalen NM (2008) The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol Lett 11:296–310
van Vuuren MMI, Robinson D, Griffiths BS (1996) Nutrient inflow and root proliferation during the exploitation of a temporally and spatially discrete source of nitrogen in soil. Plant Soil 178:185–192
Veresoglou SD, Sen R, Mamolos AP, Veresoglou DS (2011) Plant species identity and arbuscular mycorrhizal status modulate potential nitrification rates in nitrogen-limited grassland soils. J Ecol 99:1339–1349
Veresoglou SD, Chen B, Rillig MC (2012) Arbuscular mycorrhiza and soil nitrogen cycling. Soil Biol Biochem 46:53–62
Vitousek PM, Howarth RW (1991) Nitrogen limitation on land and in the sea: how can it occur? Biogeochemistry 13:87–115
Walder F, Niemann H, Natarajan M, Lehmann MF, Boller T, Wiemken A (2012) Mycorrhizal networks: common goods of plants shared under unequal terms of trade. Plant Physiol 159:789–797
Warner A, Mosse B (1980) Independent spread of vesicular-arbuscular mycorrhizal fungi in soil. Trans Br Mycol Soc 74:407–410
Weigelt A, King R, Bol R, Bardgett RD (2003) Inter-specific variability in organic nitrogen uptake of three temperate grassland species. J Plant Nutr Soil Sci 166:606–611
Welc M, Ravnskov S, Kieliszewska-Rokicka B, Larsen J (2010) Suppression of other soil microorganisms by mycelium of arbuscular mycorrhizal fungi in root-free soil. Soil Biol Biochem 42:1534–1540
Whiteside MD, Garcia MO, Treseder KK (2012) Amino acid uptake in arbuscular mycorrhizal plants. PLoS ONE 7:e47643
Wu SC, Cao ZH, Li ZG, Cheung KC, Wong MH (2005) Effects of biofertilizer containing N-fixer, P and K solubilizers and AM fungi on maize growth: a greenhouse trial. Geoderma 125:155–166
Xavier LJC, Germida JJ (2003) Selective interactions between arbuscular mycorrhizal fungi and Rhizobium leguminosarum bv. viceae enhance pea yield and nutrition. Biol Fertil Soils 37:261–267
Acknowledgments
We thank Alastair Fitter for providing helpful comments on an earlier draft of this review and the reviewers and editor for their comments. K. Storer was in receipt of a studentship from the Biotechnology and Biological Research Council (BBSRC), U.K., which is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Philippe Hinsinger.
Rights and permissions
About this article
Cite this article
Hodge, A., Storer, K. Arbuscular mycorrhiza and nitrogen: implications for individual plants through to ecosystems. Plant Soil 386, 1–19 (2015). https://doi.org/10.1007/s11104-014-2162-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-014-2162-1