Abstract
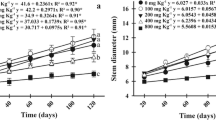
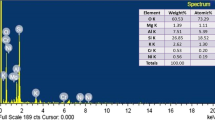
Copper uptake, localisation and biochemical and physiological traits were studied in hydroponically-grown Erica andevalensis plants at different increasing concentrations of Cu (1 µM, 50 µM, 100 µM, 250 µM, and 500 µM). Increasing Cu concentration in the nutrient medium led to a significative reduction in plant growth rate, an increase in root Cu concentration, leaf photosynthetic pigments and root peroxidase activity. Copper accumulation followed the pattern roots>stems>leaves, a typical behaviour of metal-excluders. Copper treatments led to significant changes in the free amino acid composition in shoots and roots and the concentration of polyamines in shoots. Analysis by scanning electron microscopy coupled with elemental X-ray analysis (SEM–EDX) showed a partial restriction of upward Cu transport by root vascular tissues. In leaf tissues, Cu mostly accumulated in the abaxial epidermis, suggesting a mechanism of compartmentalization to restrict mesophyll accumulation. The toxic effects of excess Cu were avoided to a certain extent by root immobilization, tissue compartmentalization, synthesis of complexing amino acids and induction of enzymes to prevent oxidative damage are among mechanisms adopted by Erica andevalensis to thrive in acidic-metalliferous soils.



Similar content being viewed by others
References
Abreu MM, Tavares MT, Batista MJ (2007) Potential use of Erica andevalensis and Erica australis in phytoremediation of sulphide mine environments: São Domingos, Portugal. J Geochem Exp 96:210–222
Asensi A, Bennett F, Brooks R, Robinson B, Stewart R (1999) Copper uptake studies on Erica. andevalensis, a metal-tolerant plant from Southwestern Spain. Commun Soil Sci Plant Anal 30:1615–1624
Banu MNA, Hoque MA, Watanabe-Sugimoto M, Matsuoka K, Nakamura Y, Shimoishi Y, Murata Y (2009) Proline and glycine betaine induce antioxidant defense gene expression and suppress cell death in cultured tobacco cells under salt stress. J Plant Physiol 166:146–156
Baszyski T, Krdl M, Krupa Z, Ruszowska M, Wojclesk AV, Wolinska D (1982) Photosynthetic apparatus of spinach exposed to excess copper. Z Pflanzenphysiol 108:385–395
Bradford MM (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye-binding. Anal Biochem 72:248–254
Cabezudo B, Rivera J (1980) Notas taxonómicas y corológicas sobre la Flora de Andalucía occidental, 2: Erica andevalensis Cabezudo & Rivera sp. nov. Lagascalia 9:223–226
Callahan DL, Baker AJM, Kolev SD, Wedd AG (2006) Metal ion ligands in hyperaccumulating plants. J Biol Inorg Chem 11:2–12
Cuypers A, Vangronsveld J, Clijsters H (2002) Peroxidase in roots and primary leaves of Phaseolus vulgaris copper and zinc phytotoxicity: a comparison. J Plant Physiol 159:869–876
Delauney AJ, Verma DPS (2003) Proline biosynthesis and osmoregulation in plants. Plant J 4:215–223
De Vos CHR, Schat H, de Waal MAM, Vooijs R, Ernst WHO (1991) Increased resistance to copper-induced damage of the root cell plasmalemma in copper tolerant Silene cucubalus. Physiol Plant 82:523–528
Ernst WHO, Verkleij JAC, Schat A (1992) Metal tolerance in plants. Acta Bot Neerl 41:229–248
Fischer RA, Turner NC (1978) Plant productivity in the arid and semiarid zones. Annu Rev Plant Phyiol 29:277–317
Hall JL (2002) Cellular mechanisms for heavy metal detoxyfication and tolerance. J Exp Bot 53:1–11
Heinrikson RL, Meredith SC (1984) Amino acid analysis by reverse-phase high-performance liquid chromatography: Precolumn derivatization with phenyliso-thiocyanate. Anal Biochem 136:65–74
Hewitt EJ (1966) Sand and water culture methods used in the study of plant nutrition. Technical Communication No. 22 (2nd revised edition). Commonwealth Bureau of Horticulture and Plantation Crops, East Malling, CAB, Farnham Royal, England
Irtelli B, Petrucci WA, Navari-Izzo F (2009) Nicotianamide and histidine/proline are, respectively, the most important copper chelators in xylem sap of Brassica carinata under conditions of copper deficiency and excess. J Exp Bot 60:260–277
Jahangir M, Abdel-Farid IB, Choi YH, Verpoorte R (2008) Metal ion-inducing metabolite accumulation in Brassica rapa. J Plant Physiol 165:1429–1437
Jiménez A, Hernández JA, de1 Rio LA, Sevilla F (1997) Evidence for the presence of the ascorbate-glutathione cycle in mitochondria and peroxisomes of pea leaves. Plant Physiol 114:275–284
Kabata-Pendias A (2001) Trace elements in soils and plants. CRC, Boca Raton
Khatun S, Babar Ali M, Hahna EJ, Paeka K-Y (2008) Copper toxicity in Withania somnifera: growth and antioxidant enzymes responses of in vitro grown plants. Environ Exp Bot 64:279–285
Kruckeberg AL, Wu L (1992) Copper tolerance and copper accumulation of herbaceous plants colonizing inactive California copper mines. Ecotox Environ Safe 23:307–319
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic membranes. Methods in Enzymology. Academic
Liu D, Kottke I (2004) Subcellular localization of copper in the root cells of Allium sativum by electron energy loss spectroscopy (EELS). Bioresource Technol 94:153–158
Macfarlane GR, Burchett MD (2001) Photosynthetic pigments and peroxidase activity as indicators of heavy metal stress in the Grey Mangrove, Avicennia marina (Forsk.) Vierh. Marine Poll Bull 42:233–240
Marcé M, Brown DS, Capell T, Figueras X, Tiburcio AF (1995) Rapid high-performance liquid chromatographic method for the quantitation of polyamines as their dansyl derivatives: application to plant and animal tissues. J Chromat B 666:329–335
Marschner H (1995) Mineral nutrition of higher plants. Academic, London
Meharg AA (2005) Mechanisms of plant resistance to metal and metalloid ions and potential biotechnological applications. Plant Soil 274:163–174
Mingorance MD, Pérez-Vázquez L, Lachica M (1993) Microwave digestion method for the atomic determination of some elements in biological samples. J Anal Atom Spectrom 8:853–858
Monni S, Salemaa M, White C, Tuittila E, Huopalainen M (2000) Copper resistance of Calluna vulgaris originating from the pollution gradient of a Cu–Ni smelter, in southwest Finland. Environ Pollut 109:211–219
Neumann D, zur Nieden U, Lichtenberger O, Leopold I (1995) How does Armeria maritima tolerate high heavy metal concentration? J Plant Physiol 146:704–717
Ouzounidou G, Čiamporová M, Moustakas M, Karataglis S (1995) Responses of maize (Zea mays L.) plants to copper stress. I. Growth, mineral content and ultrastructure of roots. Environ Exp Bot 35:167–176
Panou-Filotheou H, Bosabalidis AM (2004) Root structural aspects associated with copper toxicity in oregano (Origanum vulgare subsp. hirtum). Plant Science 166:1497–1504
Poschenrieder C, Bech J, Llugany M, Pace A, Fené E, Barceló J (2001) Copper in plant species in a copper gradient in Catalonia (North East Spain) and their potential for phytoremediation. Plant Soil 230:247–256
Quental L, Bourguignon A, Sousa AJ, Batista Brito MG, Tavares T, Abreu MM, Vairinho M, Cottard F (2002) MINEO Southern Europe environment test site, Contamination impact map** and modelling—Final Report. IST-1999-10337 http://www.brgm.fr/mineo
Robson AD, Reuter DJ (1981) Diagnosis of copper deficiency and toxiciy. In: Loneragan JF, Robson AD, Graham RD (eds) Copper in soil and plants. Academic, London, pp 287–312
Rodríguez N, Amils R, Jiménez-Ballesta R, Rufo L, De la Fuente V (2007) Heavy metal content in Erica andevalensis: an endemic plant from the extreme acidic environment of Tinto River and its soils. Arid Land Res Manag 21:51–65
Rossini Oliva S, Bargagli R, Monaci F,Valdés B, Mingorance MD, Leidi EO (2009a) Stress responses of Erica andevalensis Cabezudo & Rivera plants induced by polluted water from Tinto River (SW Spain). Ecotoxicology doi:10.1007/s10646-009-0366-6
Rossini Oliva S, Valdés B, Leidi EO (2009b) Accumulation and in vivo tissue distribution of pollutant elements in Erica andevalensis. Sci Total Environ 407:1929–1936
Salemaa M, Monni S (2003) Copper resistance of the evergreen dwarf shrub Arctostaphylos uva-ursi: an experimental exposure. Environ Pollut 126:435–443
Sharma SS, Dietz KJ (2006) The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J Exp Bot 57:711–726
Sharma SS, Dietz KJ (2009) The relationship between metal toxicity and cellular redox imbalance. Trends Plant Sci 14:43–50
Snowden RED, Wheeler BD (1995) Chemical changes in selected wetland plant species with increasing Fe supply, with specific reference to root precipitates and Fe tolerance. New Phytol 131:503–520
Soldevilla M, Maranón T, Cabrera F (1992) Heavy metal content in soil and plants from a pyrite mining area in southwest Spain. Comm Soil Plant Anal 23:1301–1319
Turnau K, Henriques FS, Anielska T, Renker C, Buscot F (2007) Metal uptake and detoxification mechanisms in Erica andevalensis growing in a pyrite mine tailing. Environ Exp Bot 61:117–123
Van Asshe F, Clijsters H (1990) Effects of metals on enzyme activity in plants. Plant Cell Environ 13:195–206
Young AJ (1991) The photoprotective role of carotenoids in higher plants. Physiol Plant 83:702–708
Acknowledgements
This research was supported by Spanish Ministry of Science and Education (MEC) (CGL2006-1418 and José Castillejo Program) and Ramón Areces Foundation. The authors thank Manlio Colella and Sergio Sorbo from CISME (Naples, Italy), for their technical assistance in SEM–EDX analysis and the techniques from the Greenhouse of Seville University for their technical assistance in plant cultivation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Juan Barcelo.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table 3 bis
Composition of the free amino acid pool in shoots and roots of Erica andevalensis plants grown in nutrient solutions containing different concentrations of Cu (in µmoles g dry weight−1). In brackets, relative values in relation to the contents in control plants (1 µM Cu). (DOC 46 kb)
Rights and permissions
About this article
Cite this article
Rossini Oliva, S., Mingorance, M.D., Valdés, B. et al. Uptake, localisation and physiological changes in response to copper excess in Erica andevalensis . Plant Soil 328, 411–420 (2010). https://doi.org/10.1007/s11104-009-0121-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-009-0121-z