Abstract
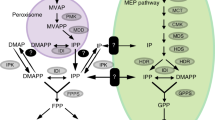
The Apocynaceae Catharanthus roseus accumulates a number of monoterpene indole alkaloids (MIAs) that originate from the coupling of the indole and the iridoid pathways. The latter pathway is usually considered as limiting for MIA biosynthesis, but evidence is now strong that the precursors tryptamine (from the indole pathway) and secologanin (from the iridoid pathway) have to be provided within the cells in a concerted manner for sustained MIA synthesis. Secologanin is formed from isopentenyl diphosphate (IPP) in a number of steps, some of which are still unknown. However significant progress has been obtained recently with the characterisation of cDNAs encoding secologanin synthase and the two constituents of geraniol 10-hydroxylase (G10H). IPP itself is formed through both the plastidial methyl-erythritol phosphate (MEP) pathway and the cytosolic mevalonate (MVA) pathway. The MEP pathway comprises 7 steps of which 4 have been identified at the molecular level in C. roseus. This pathway plays a major role in the production of MIAs, but there is now evidence that the MVA pathway serves as a minor source of precursors for iridoid biosynthesis and/or contributes (through protein prenylation) to a fine regulation of the MEP gene expression. G10H is one of the key enzymes of the MIA pathway and the up-regulation of the gene activity concomitantly with an increase in G10H activity and MIA production have been reported with various hormones and elicitors. Since regulatory genes encoding transcription factors acting on several genes of the MEP and terpenoid pathways are beginning to be characterised, metabolic engineering of the iridoid pathway could be a promising approach to control the metabolite flux towards secologanin and MIA production through biotechnological applications in the future.




Similar content being viewed by others
Abbreviations
- AACT:
-
Acetoacetyl-CoA thiolase
- ADH:
-
(monoterpene primary) Alcohol dehydrogenase
- CDP-ME:
-
4-Diphosphocytidyl-2-C-methyl-d-erythritol
- CMK:
-
4-Diphosphocytidyl-2-C-methyl-d-erythritol kinase
- CMS:
-
4-Diphosphocytidyl-2C-methyl-d-erythritol synthase
- CPR:
-
Cytochrome P450 reductase
- DL7H:
-
7-Deoxyloganin 7-hydroxylase
- DMAPP:
-
Dimethylallyl diphosphate
- DX:
-
1-Deoxy-d-xylulose
- DXP:
-
1-Deoxy-d-xylulose 5-phosphate
- DXR:
-
1-Deoxy-d-xylulose 5-phosphate reductoisomerase
- DXS:
-
1-Deoxy-d-xylulose 5-phosphate synthase
- FPP:
-
Farnesyl diphosphate
- G10H:
-
Geraniol 10-hydroxylase
- GES:
-
Geraniol synthase
- GPP:
-
Geranyl diphosphate
- GPPS:
-
Geranyl diphosphate synthase
- HDR:
-
1-Hydroxy-2-methyl-2-butenyl 4-diphosphate reductase
- HDS:
-
1-Hydroxy-2-methyl-2-butenyl 4-diphosphate synthase
- HMBPP:
-
1-Hydroxy-2-methyl-2-butenyl 4-diphosphate
- HMG-CoA:
-
3-Hydroxy-3-methylglutaryl-CoA
- HMGS:
-
3-Hydroxy-3-methylglutaryl-CoA synthase
- HMGR:
-
3-Hydroxy-3-methylglutaryl-CoA reductase
- IDI:
-
Isopentenyl diphosphate isomerase
- IPP:
-
Isopentenyl diphosphate
- LAMT:
-
Loganic acid methyltransferase
- MECS:
-
2-C-methyl-d-erythritol 2,4-cyclodiphosphate synthase
- MEP:
-
2-C-methyl-d-erythritol 4-phosphate
- MIA:
-
Monoterpene indole alkaloid
- MK:
-
Mevalonate kinase
- MVA:
-
Mevalonic acid
- MVAPP:
-
5-Diphosphomevalonate
- ORCA:
-
Octadecanoid-Responsive Catharanthus AP2/ERF-domain
- PMK:
-
Phosphomevalonate kinase
- SLS:
-
Secologanin synthase
- STR:
-
Strictosidine synthase
- TDC:
-
Tryptophan decarboxylase
References
Arigoni D, Sagner S, Latzel C, Eisenreich W, Bacher A, Zenk MH (1997) Terpenoid biosynthesis from 1-deoxy-d-xylulose in higher plants by intramolecular skeletal rearrangement. Proc Natl Acad Sci USA 94:10600–10605
Arvy MP, Imbault N, Naudascher F, Thiersault M, Doireau P (1994) 2,4-d and alkaloid accumulation in periwinkle cell suspensions. Biochimie 76:410–416
Botella-Pavia P, Besumbes O, Phillips MA, Carratero-Paulet L, Boronat A, Rodríguez-Concepción M (2004) Regulation of carotenoid biosynthesis in plants: evidence for a key role of hydroxymethylbutenyl diphosphate reductase in controlling the supply of plastidial isoprenoid precursors. Plant J 40:188–199
Bouvier F, Suire C, d’Harlingue A, Backhaus RA, Camara B (2000) Molecular cloning of geranyl diphosphate synthase and compartmentation of monoterpene synthesis in plant cells. Plant J 24:241–252
Brechbühler-Bader S, Coscia CJ, Loew P, von Szczepanski Ch, Arigoni D (1968) The chemistry and biosynthesis of loganin. Chem Commun:136–137
Bruneton J (1999) Pharmacognosie, phytochimie, plantes médicinales, Lavoisier ed, Paris, 1136 pp
Burke C, Croteau R (2002) Geranyl diphosphate synthase from Abies grandis: cDNA isolation, functional expression, and characterization. Arch Biochem Biophys 405:130–136
Burlat V, Oudin A, Courtois M, Rideau M, St-Pierre B (2004) Co-expression of three MEP pathway genes and geraniol 10-hydroxylase in internal phloem parenchyma of Catharanthus roseus implicates multicellular translocation of intermediates during the biosynthesis of monoterpene indole alkaloids and isoprenoid-derived primary metabolites. Plant J 38:131–141
Campbell M, Hahn FM, Poulter CD, Leustek T (1997) Analysis of the isopentenyl diphosphate isomerase gene family from Arabidopsis thaliana. Plant Mol Biol 36:323–328
Canel C, Lopez-Cardoso MI, Whitmer S, van der Fits L, Pasquali G, van der Heijden R, Hoge JHC, Verpoorte R (1998) Effects of over-expression of strictosidine synthase and tryptophan decarboxylase on alkaloid production by cell cultures of Catharanthus roseus. Planta 205:414–419
Canto-Canché BB, Loyola-Vargas VM (2001) Multiple forms of NADPH-cytochrome P450 oxidoreductases in the Madagascar periwinkle Catharanthus roseus. In vitro Cell Dev Biol 37:622–628
Canto-Canché BB, Meijer AH, Collu G, Verpoorte R, Loyola-Vargas VM (2005) Characterization of a polyclonal antiserum against the monoterpene monooxygenase, geraniol 10-hydroxylase from Catharanthus roseus. J. Plant Physiol 162:393–402
Chahed K, Oudin A, Guivarc’h N, Hamdi S, Chénieux JC, Rideau M, Clastre M (2000) 1-deoxy-d-xylulose-5-phosphate synthase from periwinkle: cDNA identification and induced gene expression in terpenoid indole-producing cells. Plant Physiol Biochem 38:559–566
Clastre M (1993) Purification et caractérisation de la géranyldiphosphate synthétase de cellules de Vitis vinifera L. cv. Muscat de Frontignan cultivées in vitro. Thèse d’Université, Ecole Nationale Supérieure Agronomique de Toulouse, 81 pp
Clastre M, Bantignies B, Feron G, Soler E, Ambid C (1993) Purification and characterization of geranyl diphosphate synthase from Vitis vinifera L. cv. Muscat de Frontignan cell cultures. Plant Physiol 102:205–211
Collu G, Bink HHJ, Moreno PRH, van der Heijden R, Verpoorte R (1999) Determination of the activity of the cytochrome P450 enzyme geraniol 10-hydroxylase in plants by high-performance liquid chromatography. Phytochem Anal 10:314–318
Collu G, Unver N, Peltenburg-Looman AMG, van der Heijden R, Verpoorte R, Memelink J (2001) Geraniol 10-hydroxylase, a cytochrome P450 enzyme involved in terpenoid indole alkaloid biosynthesis. FEBS Lett 508:215–220
Collu G, Garcia AA, van der Heijden R, Verpoorte R (2002) Activity of the cytochrome P450 enzyme geraniol 10-hydroxylase and alkaloid production in plant cell cultures. Plant Sci 162:165–172
Contin A, van der Heijden R, ten Hoopen HJG, Verpoorte R (1998a) The inoculum size triggers tryptamine or secologanin biosynthesis in a Catharanthus roseus cell culture. Plant Sci 139:205–211
Contin A, van der Heijden R, Lefeber AWM, Verpoorte R (1998b) The iridoid glucoside secologanin is derived from the novel triose phosphate/pyruvate pathway in a Catharanthus roseus cell culture. FEBS Lett 434:413–416
Contin A, van der Heijden R, Verpoorte R (1999a) Effects of alkaloid precursor feeding and elicitation on the accumulation of secologanin in a Catharanthus roseus cell suspension culture. Plant Cell Tiss Org Cult 56:111–119
Contin A, Collu G, van der Heijden R, Verpoorte R (1999b) The effects of phenobarbital and ketonazole on the alkaloid biosynthesis in Catharanthus roseus cell suspension cultures. Plant Physiol Biochem 37:139–144
Contin A, van der Heijden R, Verpoorte R (1999c) Accumulation of loganin and secologanin in vacuoles from suspension cultured Catharanthus roseus cells. Plant Sci 147:177–183
Courdavault V, Burlat V, St-Pierre B, Giglioli-Guivarc’h N (2005) Characterisation of CaaX-prenyl transferases in Catharanthus roseus: relationships with the expression of genes involved in the early stages of monoterpenoid biosynthetic pathway. Plant Sci 168:1097–1107
Cunningham FX Jr, Lafond TP, Gantt E (2000) Evidence of a role for LytB in the non-mevalonate pathway of isoprenoid biosynthesis. J Bacteriol 182:5841–5848
Eichinger D, Bacher A, Zenk MH, Eisenreich W (1999) Analysis of metabolic pathways via quantitative prediction of isotope labeling patterns: a retrobiosynthetic 13C NMR study on the monoterpene loganin. Phytochemistry 51:223–226
Eisenreich W, Rodhich F, Bacher A (2001) Deoxyxylulose phosphate pathway to terpenoids. Trends Plant Sci 6:78–84
Estevez JM, Cantero A, Reindl A, Reichler S, León P (2001) 1-deoxy-d-xylulose-5-phosphate synthase, a limiting enzyme for plastidic isoprenoid biosynthesis in plants. J Biol Chem 276:22901–22909
Facchini PJ, DiCosmo F (1991) Secondary metabolite biosynthesis in cultured cells of Catharanthus roseus (L) G Don immobilized by adhesion to glass fibres. Appl Microbiol Biotechnol 35:382–392
Galichet A, Fuerholz A, Laule O, Beemster G, Gruissem W (2004) Developmental coordination of MVA and MEP isoprene pathways requires protein prenylation. In: Conferences Jacques-Monod: Integrative Biology, dissecting cross talk between plant signalling pathway, 15–19 May 2004, Abstract 9
Gantet P, Imbault N, Thiersault M, Doireau P (1998) Necessity of a functional octodecanoic pathway for indole alkaloid synthesis by Catharanthus roseus cell suspensions cultured in an auxin-starved medium. Plant Cell Physiol 39:220–225
Gantet P, Memelink J (2002) Transcription factors: tools to engineer the production of pharmacologically active plant metabolites. Trends Pharmacol Sci 23:563–569
Guarnaccia R, Coscia CJ (1971) Occurrence and biosynthesis of secologanic acid in Vinca rosea. J Am Chem Soc 93:6320–6321
Guevara-García A, San Román C, Arroyo A, Cortés ME, de la Luz Gutiérrez-Nava M, León P (2005) Characterization of the Arabidopsis clb6 mutant illustrates the importance of post transcriptional regulation of the methyl-d-erythritol 4-phosphate pathway. Plant Cell 17:628–643
Hallahan DL, West JM, Wallsgrove RM, Smiley DW, Dawson GW, Pickett JA, Hamilton JG (1995) Purification and characterization of an acyclic monoterpene primary alcohol: NADP+ oxidoreductase from catmint (Nepeta racemosa). Arch Biochem Biophys 318:105–112
Hallard D, van der Heijden R, Contin A, Tomas Jiménéz EM, Snoeijer W, Verpoorte R, Jensen SR, Lopez Cardoso MI, Pasquali G, Memelink J, Hoge JHC (1998) An assay for secologanin in plant tissues based on enzymatic conversion into strictosidine. Phytochem Anal 9:162–167
Hedhili S, Courdavault V, Giglioli-Guivarc’h N, Gantet P (2005) Regulation of the terpene moiety biosynthesis of Catharanthus roseus terpene indole alkaloids. Phytochem Rev this issue
Hong SB, Hughes EH, Shanks JV, San KY, Gibson SI (2003) Role of the nonmevalonate pathway in indole alkaloid production by Catharanthus roseus hairy roots. Biotechnol Prog 19:1105–1108
Hughes EH, Hong SB, Gibson SI, Shanks JV, San KY (2004a) Metabolic engineering of the indole pathway in Catharanthus roseus hairy roots and increased accumulation of tryptamine and serpentine. Metab Eng 6:268–276
Hughes EH, Hong SB, Gibson SI, Shanks JV, San KY (2004b) Expression of a feedback-resistant anthranilate synthase in Catharanthus roseus hairy roots provides evidence for tight regulation of terpenoid indole levels. Biotechnol Bioeng 86:718–727
Iijima Y, Davidovich-Rikanati R, Fridman E, Gang DR, Bar E, Lewinsohn E, Pichersky E (2004) The biochemical and molecular basis for the divergent patterns in the biosynthesis of terpenes and phenylpropenes in the peltate glands of three cultivars of basil. Plant Physiol 136:3724–3736
Ikeda H, Esaki N, Nakai S, Hashimoto K, Uesato S, Soda K, Fujita T (1991) Acyclic monoterpene primary alcohol: NADP+ oxidoreductase of Rauwolfia serpentina cells: the key enzyme in biosynthesis of monoterpene alcohols. J Biochem 109:341–347
Imbault N, Thiersault M, Dupéron P, Benabdelmouna A, Doireau P (1996) Pravastatin: a tool for investigating the availability of mevalonate metabolites for primary and secondary metabolism in Catharanthus roseus cell suspensions. Physiol Plant 98:803–809
Irmler S, Schröder G, St-Pierre B, Crouch NP, Hotze M, Schmidt J, Strack D, Matern U, Schröder J (2000) Indole alkaloid biosynthesis in Catharanthus roseus: new enzyme activities and identification of cytochrome P450 CYP72A1 as secologanin synthase. Plant J 24:797–804
Jacobs DI, Gaspari M, van der Greet J, van der Heijden R, Verpoorte R (2005) Proteome analysis of the medicinal plant Catharanthus roseus. Planta 221:690–704
Katano N, Yamamoto H, Ilio R, Inoue K (2001) 7-deoxyloganin 7-hydroxylase in Lonicera japonica cell cultures. Phytochemistry 58:53–58
Krepkiy D, Miziorko HM (2004) Identification of active site residues in mevalonate diphosphate decarboxylase: implications for a family of phosphotransferases. Protein Sci 13:1875–1881
Krueger RJ, Carew DP (1978) Catharanthus roseus tissue culture: the effects of precursors on growth and alkaloid production. Lloydia 41:327–331
Laule O, Fürholz A, Chang H-S, Zhu T, Wang X, Heifetz PB, Gruissem W, Lange BM (2003) Crosstalk between cytosolic and plastidial pathways of isoprenoid biosynthesis in Arabidopsis thaliana. Proc Natl Acad Sci U S A 100:6866–6871
Leivar P, Gonzalez VM, Castel S, Trelease RN, Lopez-Iglesias C, Arro M, Boronat A, Campos N, Ferrer A, Fernandez-Busquets X (2005) Subcellular localization of Arabidopsis 3-hydroxy-3-methylglutaryl-Coenzyme A reductase. Plant Physiol 137:57–69
Lois LM, Rodríguez-Concepción M, Gallego F, Campos N, Boronat A (2000) Carotenoid biosynthesis during tomato fruit development: regulatory role of 1-deoxy-d-xylulose 5-phosphate synthase. Plant J 22:503–513
Lopez Cardoso MI, Meijer AH, Rueb S, Machado JA, Memelink J, Hoge JH (1997) A promoter region that controls basal and elicitor-inducible expression level of the NADPH:cytochrome P450 reductase gene (Cpr) from Catharanthus roseus binds nuclear factor GT-1. Mol Gen Genet 256:674–681
Madyastha KM, Guarnaccia R, Baxter C, Coscia CJ (1973) S-adenosyl-l-methionine: loganic acid methyltransferase. A carboxyl-alkylating enzyme from Vinca rosea. J Biol Chem 248:2497–2501
Madyastha KM, Coscia CJ (1979) Detergent-solubilized NADPH-cytochrome c (P450) reductase from the higher plant, Catharanthus roseus. J Biol Chem 254:2419–2427
McFarlane J, Madyastha KM, Coscia CJ (1975) Regulation of secondary metabolism in higher plants. Effect of alkaloids on a cytochrome P450 dependent monooxygenase. Biochem Biophys Res Commun 66:1263–1269
Mahroug S, Burlat V, St-Pierre B (2005) Cellular and sub-cellular organisation of the monoterpenoid indole alkaloid pathway in Catharanthus roseus. Phytochem Rev this issue
Maldonado-Mendoza IE, Burnett RJ, Nessler CL (1992) Nucleotide sequence of a cDNA encoding 3-hydroxy-3-methylglutaryl coenzyme A reductase. Plant Physiol 100:1613–1614
Meehan TD, Coscia CJ (1973) Hydroxylation of geraniol and nerol by a monooxygenase from Vinca rosea. Biochem Biophys Res Commun 53:1043–1048
Meijer AH, Lopez Cardoso MI, Voskuilen JTH, de Waal A, Verpoorte R, Hoge JHC (1993a) Isolation and characterization of a cDNA clone from Catharanthus roseus encoding NADPH: cytochrome P450 reductase, an essential enzyme for reactions catalysed by cytochrome P450 monooxygenases in plants. Plant J 4:47–60
Meijer AH, de Waal A, Verpoorte R (1993b) Purification of the cytochrome P450 enzyme geraniol 10-hydroxylase from cell cultures of Catharanthus roseus. J Chromatogr 635:237–249
Mérillon JM, Doireau P, Guillot A, Chénieux JC, Rideau M (1986) Indole alkaloid accumulation and tryptophan decarboxylase activity in Catharanthus roseus cells cultured in three different media. Plant Cell Rep 5:23–26
Mérillon JM, Ouelhazi L, Doireau P, Chénieux JC, Rideau M (1989) Metabolic changes and alkaloid production in habituated and non-habituated cells of Catharanthus roseus grown in hormone-free medium. Comparing hormone-deprived non-habituated cells with habituated cells. J Plant Physiol 134:54–60
Mincheva Z, Courtois M, Crèche J, Rideau M, Viaud-Massuard MC (2004) One pot synthesis of functionalized 4,5-dihydroisoxazole derivatives via nitrile oxides, and biological evaluation with plant cells. Bioorg Med Chem 12:191–197
Moreno PRH, van der Heijden R, Verpoorte R (1993a) Effect of terpenoid precursor feeding and elicitation on formation of indole alkaloids in cell suspension cultures of Catharanthus roseus. Plant Cell Rep 12:702–705
Moreno PRH, Schlatmann JE, van der Heijden R, van Gulik WM, ten Hoopen HJ, Verpoorte R, Heijnen JJ (1993b) Induction of ajmalicine formation and related enzyme activities in Catharanthus roseus cells: effect of inoculum density. Appl Microbiol Biotech 39:42–47
Moreno PRH, Poulsen C, van der Heijden R, Verpoorte R (1996) Effects of elicitation on different metabolic pathways in Catharanthus roseus (L.) G. Don cell suspension cultures. Enzyme Microb Technol 18:99–107
Morgan JA, Shanks JV (2000) Determination of metabolic rate-limitations by precursor feeding in Catharanthus roseus hairy root cultures. J Biotechnol 79:137–145
Nagata N, Suzuki M, Yoshida S, Muranaka T (2002) Mevalonic acid partially restores chloroplast and etioplast development in Arabidopsis lacking the non-mevalonate pathway. Planta 216:345–350
Nakamura A, Shimada H, Masuda T, Ohta H, Takamiya KI (2001) Two distinct isopentenyl diphosphate isomerases in cytosol and plastid are differentially induced by environmental stresses in tobacco. FEBS Lett 506:61–64
Page JE, Hause G, Raschke M, Gao W, Schmidt J, Zenk MH, Kutchan TM (2004) Functional analysis of the final steps of the 1-deoxy-d-xylulose (DXP) pathway to isoprenoids in plants using virus-induced gene silencing. Plant Physiol 134:1401–1413
Papon N, Bremer J, Vansiri A, Andreu F, Rideau M, Crèche J (2005) Cytokinin and ethylene control indole alkaloid production at the level of the MEP/terpenoid pathway in Catharanthus roseus suspension cells. Planta Med 71:572–574
Proteau PJ (2004) 1-deoxy-d-xylulose 5-phosphate reductoisomerase: an overview. Bioorg Chem 32:483–493
Querol J, Campos N, Imperial S, Boronat A, Rodríguez-Concepción M (2002) Functional analysis of the Arabidopsis thaliana GCPE protein involved in plastid isoprenoid biosynthesis. FEBS Lett 514:343–346
Ramos-Valdivia AC (1996) Characterization of isopentenyl diphosphate isomerase. A regulatory enzyme in Cinchona isoprenoid biosynthesis, PhD Thesis, Leiden University, The Netherlands, 145 pp
Ramos-Valdivia AC, van der Heijden R, Verpoorte R (1998) Isopentenyl diphophate isomerase and prenyltransferase activities in rubiaceous and apocynaceous cultures. Phytochemistry 48:961–969
Richard SB, Ferrer JL, Bowman ME, Lillo AM, Tetzlaff CN, Cane DE, Noel JP (2002) Structure and mechanism of 2-C-methyl-d-erythritol 2,4-cyclodiphosphate synthase. An enzyme in the mevalonate-independent isoprenoid biosynthetic pathway. J Biol Chem 277:8667–8672
Riou C, Tourte Y, Lacroute F, Karst F (1994) Isolation and characterization of a cDNA encoding Arabidopsis thaliana mevalonate kinase by genetic complementation in yeast. Gene 148:293–297
Rodríguez-Concepción M, Ahumada I, Diez-Juez E, Sauret-Gueto S, Lois LM, Gallego F, Carratero-Paulet L, Campos N, Boronat A (2001) 1-deoxy-xylulose 5-phosphate reductoisomerase and plastid isoprenoid biosynthesis during tomato fruit ripening. Plant J 27:213–222
Rodríguez-Concepción M, Boronat A (2002) Elucidation of the methylerythritol phosphate pathway for isoprenoid biosynthesis in bacteria and plastids. A metabolic milestone achieved through genomics. Plant Physiol 130:1079–1089
Rodríguez-Concepción M, Querol J, Lois LM, Imperial S, Boronat A (2003) Bioinformatic and molecular analysis of hydroxymethylbutenyl diphosphate synthase (GCPE) gene expression during carotenoid accumulation in ripening tomato fruit. Planta 217:476–482
Rodríguez-Concepción M, Forés O, Martinez-Garcia JF, Gonzalez V, Phillips MA, Ferrer A, Boronat A (2004) Distinct light-mediated pathways regulate the biosynthesis and exchange of isoprenoid precursors during Arabidopsis development. Plant Cell 16:144–156
Rohmer M (1999) The discovery of a mevalonate-independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants. Nat Prod Rep 16:565–574
Sanchez-Iturbe P, Galaz-Avalos RM, Loyola-Vargas VM (2005) Determination and partial purification of a monoterpene cyclase from Catharanthus roseus hairy roots. Phyton 55–69
Schiel O, Witte L, Berlin J (1987) Geraniol-10-hydroxylase activity and its relation to monoterpene indole alkaloid accumulation in cell suspension cultures of Catharanthus roseus. Z Naturforsch 42:1075–1081
Schuhr CA, Radykewicz T, Sagner S, Latzel C, Zenk MH, Arigoni D, Bacher A, Rohdich F, Eisenreich W (2003) Quantitative assessment of cross talk between the two isoprenoid biosynthesis pathways in plants by NMR spectroscopy. Phytochem Rev 2:3–16
Schulte AE, van der Heijden R, Verpoorte R (1999) Purification and characterization of phosphomevalonate kinase from Catharanthus roseus. Phytochemistry 52:975–983
Schulte AE, van der Heijden R, Verpoorte R (2000a) Purification and characterization of mevalonate kinase from suspension-cultured cells of Catharanthus roseus (L.) G. Don. Arch Biochem Biophys 378:287–298
Schulte AE, Llamas Duran EM, van der Heijden R, Verpoorte R (2000b) Mevalonate kinase activity in Catharanthus roseus plants and suspension cultured cells. Plant Sci 150:59–69
Scott AI, Mizukami H, Lee SL (1979) Characterization of a 5-methyltryptophan resistant strain of Catharanthus roseus cultured cells. Phytochemistry 18:795–798
Seemann M, Wegner P, Schünemann V, Tse Sum Bui B, Wolff M, Marquet A, Trautwein AX, Rohmer M (2005) Isoprenoid biosynthesis in chloroplasts via the methylerythritol phosphate pathway: the (E)-4-hydroxy-3-methylbut-2-enyl diphosphate synthase (GcpE) from Arabidopsis thaliana is a [4Fe–4S] protein. J Biol Inorg Chem 10:131–137
Soler E, Clastre M, Bantignies B, Marigo G, Ambid C (1993) Uptake of isopentenyl diphosphate by plastids isolated from Vitis vinifera L. cell suspensions. Planta 191:324–329
Tholl D, Kish CM, Orlova I, Sherman D, Gershenzon J, Pichersky E, Dudareva N (2004) Formation of monoterpenes in Antirrhinum majus and Clarkia breweri flowers involves heterodimeric geranyl diphosphate synthases. Plant Cell 16:977–992
Uesato S, Matsuda S, Inouye H (1984) Mechanism for iridane skeleton formation from acyclic monoterpenes in the biosynthesis of secologanin and vindoline in Catharanthus roseus and Lonicera morrowii. Chem Pharm Bull 23:1671–1674
Uesato S, Ogawa Y, Inouye H, Saiki K, Zenk MH (1986a) Synthesis of iridodial by cell free extracts from Rauwolfia serpentina. Tetrahedron Lett 27:2893–2896
Uesato S, Miyauchi M, Itoh H, Inouye H (1986b) Biosynthesis of iridoid glucosides in Galium mollugo, G. spurium, var echinospermum and Deutzia crenata. Intermediacy of deoxyloganic acid, loganin and iridodial glucoside. Phytochemistry 25:2515–2521
Uesato S, Ikeda H, Fujita T, Inouye H, Zenk MH (1987) Elucidation of iridodial formation mechanism. Partial purification and characterisation of the novel monoterpene cyclase from Rauwolfia serpentina cell suspension cultures. Tetrahedron Lett 28:4431–4434
Van der Fits L, Memelink J (2000) ORCA3, a jasmonate-responsive transcriptional regulator of plant primary and secondary metabolism. Science 289:295–297
Van der Heijden R, Verpoorte R (1995) Metabolic enzymes of 3-hydroxy-3-methylglutaryl-coenzyme A in Catharanthus roseus. Plant Cell Tiss Org Cult 43:85–88
Van der Heijden R, Verpoorte R, Duine JA (1994) Biosynthesis of 3S-hydroxy-3-methylglutaryl-coenzyme A in Catharanthus roseus: acetoacetyl-CoA thiolase and HMG-CoA synthase show similar chromatographic behaviour. Plant Physiol Biochem 32:807–812
Van der Heijden R, Jacobs DI, Snoeijer W, Hallard D, Verpoorte R (2004) The Catharanthus roseus alkaloids: pharmacognosy and biotechnology. Curr Med Chem 11:607–628
Veau B, Courtois M, Oudin A, Chénieux JC, Rideau M, Clastre M (2000) Cloning and expression of cDNA encoding two enzymes of the MEP pathway in Catharanthus roseus. Biochim Biophys Acta 1517:159–163
Vetter H-P, Mangold U, Schroeder G, Marner F-J, Werck-Reichhart D, Schroerder J (1992) Molecular analysis and heterologous expression of an inducible cytochrome P-450 protein from periwinkle (Catharanthus roseus L.). Plant Physiol 100:998–1007
Walter MH, Hans J, Strack D (2002) Two distantly related genes encoding 1-deoxy-d-xylulose 5-phosphate synthases: differential regulation in shoots and apocarotenoid-accumulating mycorrhizal roots. Plant J 31:243–254
Whitmer S, van der Heijden R, Verpoorte R (2002a) Effect of precursor feeding on alkaloid accumulation by a tryptophan decarboxylase over-expressing transgenic cell line T22 of Catharanthus roseus. J Biotechnol 96:193–203
Whitmer S, van der Heijden R, Verpoorte R (2002b) Effect of precursor feeding on alkaloid accumulation by a strictosidine synthase over-expressing transgenic cell line S1 of Catharanthus roseus. Plant Cell Tiss Org Cult 69:85–93
Wolfertz M, Sharkey TD, Boland W, Kühnemann F (2004) Rapid regulation of the methylerythritol 4-phosphate pathway during isoprene synthesis. Plant Physiol 135:1939–1945
Wolff M, Seemann M, Tse Sum Bui B, Frapart Y, Tritsch D, Estrabot AG, Rodríguez-Concepción M, Boronat A, Marquet A, Rohmer M (2003) Isoprenoid biosynthesis via the methylerythritol phosphate pathway: the (E)-4-hydroxy-3-methylbut-2-enyl diphosphate reductase (LytB/IspH) from Escherichia coli is a [4Fe–4S] protein. FEBS Lett 541:115–120
Yang T, Li J, Wang HX, Zeng Y (2005) A geraniol-synthase gene from Cinnamomum tenuipilum. Phytochemistry 66:285–293
Zhao J, Hu Q, Guo Y-Q, Zhu W-H (2001) Effects of stress factors, bioregulators, and synthetic precursors on indole alkaloid production in compact callus clusters cultures of Catharanthus roseus. Appl Microbiol Biotechnol 55:693–698
Zenk MH, El-Saghi H, Arens H, Stockkigt J, Weiler EW, Deus B (1977) Formation of the indole alkaloids serpentine and ajmalicine in cell suspension cultures of Catharanthus roseus. In: Barz W, Reinhard E, Zenk MH (eds) Plant tissue culture and its biotechnological application. Springer Verlag, Berlin, New York, pp 27–43
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oudin, A., Courtois, M., Rideau, M. et al. The iridoid pathway in Catharanthus roseus alkaloid biosynthesis. Phytochem Rev 6, 259–276 (2007). https://doi.org/10.1007/s11101-006-9054-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11101-006-9054-9