Abstract
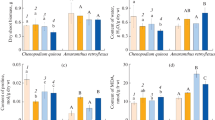
The aim of this study was to evaluate the effects of low air temperature during nocturnal (TN) and diurnal (TD) periods as well as the substrate temperature (TS) on photosynthesis of ‘Valencia’ orange tree grafted on Rangpur lime rootstock. The experiment was carried out in a growth chamber with seven-month-old plants. The plants were exposed to the following temperature regimes: low substrate temperature (LTS, with: TD = 28°C, TN = 20°C, TS = 10°C); low air temperature during night (LTN, with: TD = 28°C, TN = 10°C, TS = 26°C); low temperature during nighttime and also low substrate temperature (LTSN, with: TD = 28°C, TN = 10°C, TS = 10°C); low air temperature during both diurnal and nocturnal periods (LTND, with: TD = 17°C, TN = 10°C, TS = 26°C); and finally to low air temperature (night and day) and low substrate temperature (LTSND, with: TD = 17°C, TN = 10°C, TS = 10°C). As reference (control), plants were subjected to TD = 28°C, TN = 20°C, and TS = 26°C. Measurements of leaf gas exchange, photochemical activity and carbohydrate concentrations were performed after six days of exposure to each thermal treatment. Compared to the control, all thermal regimes caused reductions in photosynthesis due to diffusive and metabolic limitations. The photoinhibition was transient in plants exposed to night and substrate low temperatures, whereas it was severe and chronic in plants subjected to chilling during the diurnal period. However, the lowest photosynthesis was observed in plants with low substrate temperature of 10°C (in LTS, LTSND and LTSN treatments), regardless of air temperature. The occurrence of cold night and/or its combination with low substrate temperature caused accumulation of starch in leaves. When considering carbohydrate concentrations in stems and roots, it was not possible to establish a clear response pattern to chilling. In conclusion, the low substrate temperature causes a greater reduction of CO2 assimilation in citrus plants as compared to the occurrence of low air temperature, being such response a consequence of diffusive and biochemical limitations.
Similar content being viewed by others
Abbreviations
- C:
-
control
- C C :
-
CO2 concentration in chloroplast
- C i :
-
intercellular CO2 concentration
- g m :
-
mesophyll conductance
- g s :
-
stomatal conductance
- ETR:
-
apparent electron transport rate
- FV/FM :
-
maximum quantum efficiency of PSII
- ΔF/FM′:
-
effective quantum efficiency of PSII
- LTN :
-
low nocturnal air temperature
- LTND :
-
low air temperature during both nocturnal and diurnal periods
- LTS :
-
low substrate temperature
- LTSN :
-
low substrate temperature and low nocturnal air temperature
- LTSND :
-
low substrate temperature and cold conditions during both diurnal and nocturnal periods
- NPQ:
-
nonphotochemical quenching
- P N :
-
leaf net CO2 assimilation rate
- P NI :
-
diurnal CO2 assimilation
- PPFD:
-
photosynthetic photon flux density
- PSII:
-
photosystem II
- qP :
-
photochemical quenching
- TD :
-
diurnal air temperature
- TN :
-
nocturnal air temperature
- TS :
-
substrate temperature
References
Allen, D.J., Ort, D.R.: Impacts of chilling temperatures on photosynthesis in warm-climate plants. — Trends Plant Sci. 6: 36–42, 2001.
Allen, D.J., Ratner, K., Giller, Y.E., Gussakovsky, E.E., Shahak, Y., Ort, D.R.: An overnight chill induces a delayed inhibition of photosynthesis at midday in mango (Manguifera indica L.). — J. Exp. Bot. 51: 1893–1902, 2000.
Amaral, L.I.V., Gaspar, M., Costa, P.M.F., Aidar, M.P.M., Buckeridge, M.S.: [A new rapid and sensitive enzymatic method for extraction and quantifi cation of starch in plant material.] — Hoehnea 34: 425–431, 2007.[In Portuguese.]
Asada, K.: The water-water cycle as alternative photon and electron sinks. — Philos. T. Roy. Soc. B. 355: 1419–1431, 2000.
Bevington, B.K., Castle, W.S.: Annual root growth pattern of young citrus trees in relation to shoot growth, soil temperature, and soil water content. — J. Am. Soc. Hort. Sci. 110: 840–845, 1985.
Bieleski, R.L., Turner, A.: Separation and estimation of amino acids in crude plant extracts by thin-layer electrophoresis and chromatography. — Anal. Biochem. 17: 278–293, 1966.
Castle, W.S.: Citrus root systems: their structure, function, growth and relationship to tree performance. — Proc. Int. Soc. Citriculture 62: 62–69, 1980.
Critchley, C.: Photoinhibition. — In: Raghavendra, A.S. (ed.): Photosynthesis: A Comprehensive Treatise. Pp. 264–272. Cambridge Univ. Press, Cambridge 1998.
Dubois, M., Gilles, K.A., Hamilton, J.K., Rebers, P.A., Smith, F.: Colorimetric method for determination of sugars and related substances. — Anal. Chem. 28: 350–356, 1956.
Erismann, N.M., Machado, E.C., Tuccci, M.L.S.: Photosynthetic limitation by CO2 diffusion in drought stressed orange leaves on tree rootstocks. — Photosynth. Res. 96: 163–172, 2008.
Foyer, C.H.: Feedback inhibition of photosynthesis through source-sink regulation in leaves. — Plant Physiol. Bioch. 26: 483–492, 1988.
Goldschmidt, E.E., Golomb, A.: The carbohydrate balance of alternate-bearing citrus trees and the significance of reserves for flowering and fruiting. — J. Amer. Soc. Hort. Sci.: 107: 206–208, 1982.
Goldschmidt, E.E., Koch, K.E.: Citrus. — In: Zamski, E.; Schaffer, A.A. (ed.) Photoassimilate Distribution in Plants and Crops. Source-Sink Relationships. Pp. 797–823. Marcel Dekker, Inc., New York 1996.
Habermann, G., Rodrigues, J.D.: Leaf gas exchange and fruit yield in sweet orange trees as affected by Citrus Variegated Chlorosis and environmental conditions. — Sci. Hort. 122: 69–76, 2009.
Handel, E.V.: Direct microdetermination of sucrose. — Anal. Biochem. 22: 280–283, 1968.
Harley, P.C., Loreto, F., Di Marco, G., Sharkey, T.D.: Theoretical considerations when estimating mesophyll conductance to CO2 flux by analysis of the response of photosynthesis to CO2. — Plant Physiol. 98: 1429–1436, 1992.
Iglesias, D.J.; Lliso, I.; Tadeo, F.R.; Talon, M.: Regulation of photosynthesis through source-sink imbalance in citrus is mediated by carbohydrate content in leaves. — Physiol. Plant. 116: 563–572, 2002.
Kramer, P.J.: Water Relations of Plants. — Academic Press, New York 1983.
Lee, H.S., Chung, G.C., Steudle, E.: Gating of aquaporins by low temperature in roots of chilling-sensitive cucumber and chilling-tolerant figleaf gourd. — J. Exp. Bot. 56: 985–995, 2005.
Long, S.P., Humphries, S., Falkowski, P.G.: Photoinhibition of photosynthesis in nature. — Annu. Rev. Plant. Phys. 45: 633–661, 1994.
Machado, D.F.S.P., Machado, E.C., Machado, R.S., Ribeiro, R.V.: [Effects of low night temperature and rootstocks on diurnal variation of leaf gas exchange rates and photochemical activity of ‘Valência’ sweet orange plants.] — Rev. Bras. Frut. 32: 351–359, 2010. [In Portuguese.]
Machado, E.C., Medina, C.L., Gomes, M.M.A., Habermann, G.: [Seasonal variation of photosynthetic rates, stomatal conductance and leaf water potential in ‘Valencia’ orange trees.] — Sci. Agri. 59: 53–58, 2002. [In Portuguese.]
Machado, E.C., Schmidt, P.T., Medina, C.L., Ribeiro, R.V.: [Photosynthetic responses of three citrus species to environmental factors.] — Pesq. Agropec. Bras. 40: 1161–1170, 2005. [In Portuguese.]
Magalhães Filho, J.R., Machado, E.C., Machado, D.F.S.P. Ramos, R.A., Ribeiro, R.V.: [Root temperature variation and photosynthesis of ‘Valencia’ sweet orange nursery trees.] — Pesq. agropec. Bras. 44: 1118–1126, 2009. [In Portuguese.]
Maurel, C., Verdoucq, L., Luu, D., Santoni, V.: Plant aquaporins: membrane channels with multiple integrated functions. — Annu. Rev. Plant Biol. 59: 595–624, 2008.
Medina, C.L., Souza, R.P., Machado, E.C., Ribeiro, R.V., Silva, J.A.B.: Photosynthetic response of citrus grown under reflective aluminized polypropylene shading nets. — Sci. Hort. 96: 115–125, 2002.
Osmond, C.B.; Grace, S.C.: Perspectives on photoinhibition and photorespiration in the field: quintessential inefficiencies of the light and dark reactions of photosynthesis? — J. Exp. Bot. 46: 1351–1362, 1995.
Paul, M.J.; Foyer, C.H.: Sink regulation of photosynthesis. — Exp. Bot. 52: 1383–1400, 2001.
Ramos, R. A., Ribeiro, R. V., Machado, E. C., Machado, R. S.: [Seasonal variation in vegetative growth of Hamlin sweet orange grafted on Swingle citrumelo plants, in Limeira, São Paulo State.] — Acta Scientiarum Agron. 32: 537–545, 2010. [In Portuguese.]
Reuther, W. Citrus.: — In: Alvim, P.T.; Kozlowski, T.T. Ecophysiology of Tropical Crops. Pp. 409–439. Academic Press, London 1977.
Ribeiro, R.V.: [Seasonal variation of photosynthesis and water relations of ‘Valencia’ sweet orange plants.] — PhD. Thesis, Pp. 156. Universidade São Paulo, Piracicaba 2006. [In Portuguese.]
Ribeiro, R.V., Machado, E.C. Some aspects of citrus ecophysiology in subtropical climates: re-visiting photosynthesis under natural conditions. — Braz. J. Plant Physiol. 19: 393–411, 2007.
Ribeiro, R.V., Machado, E.C., Brunini, O.: Occurrence of environmental conditions for flowering induction of sweet orange plants in the State of São Paulo. — Rev. Bras. Frutic. 28: 247–253, 2006. [In Portuguese.]
Ribeiro, R.V., Machado, E.C., Santos, M.G., Oliveira, R.F.: Photosynthesis and water relations of well-watered orange plants as affected by winter and summer conditions. — Photosynthetica 47: 215–222, 2009a.
Ribeiro, R.V., Machado, E.C., Santos, M.G., Oliveira, R.F.: Seasonal and diurnal changes in photosynthetic limitation of young sweet orange trees. — Environ. Exp. Bot. 66: 203–211, 2009b.
Roháček, K.: Chlorophyll fluorescence parameters: the definitions, photosynthetic meaning, and mutual relationships. — Photosynthetica 40: 13–29, 2002.
Tournaire-Roux, C., Sutka, M.; Javot, H., Gout, E., Gerbeau, P., Luu, D.T., Bligny, R. Maurel, C.: Cytosolic pH regulates root water transport during anoxic stress through gating of aquaporins. — Nature 425: 393–397, 2003.
Veselova, S.V., Farhutdinov, R.G., Veselov, S.Y., Kudoyarova, G.R., Veselov, D.S., Hartung, W.: The effect of root cooling on hormone content, leaf conductance and root hydraulic conductivity of durum wheat seedlings (Triticum durum L.). — J. Plant Physiol. 162: 21–16, 2005.
Zhang, Y.P., Qiao, Y.X., Zhang, Y.L., Zhou, Y.H., Yu, J.Q.: Effects of root temperature on leaf gas exchange and xylem sap abscisic acid concentrations in six Cucurbitaceae species. — Photosynthetica 46: 356–362, 2008.
Zhou, Y., Huang, L., Zhang, Y., Shi, K., Yu, J., Nogue, S.: Chill-induced decrease in capacity of RuBP carboxylation and associated H2O2 accumulation in Cucumber leaves are alleviated by grafting onto Figleaf Gourd. — Ann. Bot. 100: 839–848, 2007.
Acknowledgments
The authors gratefully acknowledge the Conselho Nacional de Desenvolvimento Científico e Tecnologico (CNPq, Brazil), the Fundação de Amparo à Pesquisa do Estado de São Paulo (Fapesp, Brazil) for fellowships (RVR and ECM) and scholarships (CMAS, DFSPM and JMRF) granted and also to Fapesp for funding this research (05/57862-8).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Santos, C.M.A., Ribeiro, R.V., Magalhães Filho, J.R. et al. Low substrate temperature imposes higher limitation to photosynthesis of orange plants as compared to atmospheric chilling. Photosynthetica 49, 546–554 (2011). https://doi.org/10.1007/s11099-011-0071-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11099-011-0071-6