Abstract
Purpose
Ethambutol (EMB) is a first-line anti-tubercular drug that is known to cause optic neuropathy. The exact mechanism of its eye toxicity is unknown; however, proposition is metal chelating effect of both EMB and its metabolite 2,2'-(ethylenediamino)-dibutyric acid (EDBA). The latter is formed by sequential metabolism of EMB by alcohol dehydrogenases (ADHs) and aldehyde dehydrogenases (ALDHs). The purpose of this study was to predict the levels of drug and EDBA in the eye using physiologically based pharmacokinetic (PBPK) modeling.
Methods
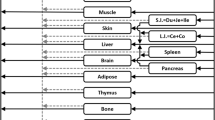
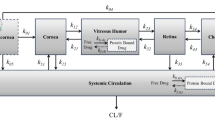
The PBPK model of EMB was developed using GastroPlus. The intrinsic hepatic clearance of ALDH, calculated by the model, was scaled down using proteomics data to estimate the rate of formation of EDBA in the eye. Additionally, the comparative permeability of EMB and EDBA was assessed by employing in silico and in vitro approaches. The rate of formation of EDBA in the eye and permeability data were then incorporated in a compartmental model to predict the ocular levels of EMB and EDBA.
Results
The simulation results of compartmental model highlighted that there was an on-site formation of EDBA upon metabolism of EMB. Furthermore, in silico and in vitro studies revealed that EDBA possessed much lower permeability than EMB. These observations meant that once EDBA was formed in the eye, it was not permeated out and hence achieved higher ocular concentration.
Conclusion
The on-site formation of EDBA in the eye, its higher local concentration due to lower ocular clearance and its pre-known characteristic to chelate metal species better explains the ocular toxicity shown by EMB.





Similar content being viewed by others
References
WHO. Ethambutol efficacy and toxicity: literature review and recommendations for daily and intermittent dosage in children. Geneva, Switzerland: World Health Organization; 2006.
WHO. Stop TB initiative: treatment of tuberculosis: guidelines. Geneva, Switzerland: World Health Organization; 2010.
Addington WW. The side effects and interactions of anti-tuberculosis drugs. Chest. 1979;76(6):782–4.
Alvarez KL, Krop LC. Ethambutol-induced ocular toxicity revisited. Ann Pharmacother. 1993;27(1):102–3.
Chatterjee V, Buchanan D, Friedmann A, et al. Ocular toxicity following ethambutol in standard dosage. Br J Dis Chest. 1986;80:288–91.
Citron K, Thomas G. Ocular toxicity from ethambutol. Thorax. 1986;41(10):737.
De Palma P, Franco F, Bragliani G, et al. The incidence of optic neuropathy in 84 patients treated with ethambutol. Metab Pediatr Syst Ophthalmol. 1989;12(1–3):80–2.
Estlin KAT, Sadun AA. Risk factors for ethambutol optic toxicity. Int Ophthalmol. 2010;30(1):63–72.
Garg P, Garg R, Prasad R, et al. A prospective study of ocular toxicity in patients receiving ethambutol as a part of directly observed treatment strategy therapy. Lung India. 2015;32(1):16.
Griffith DE, Brown Elliott BA, Shepherd S, et al. Ethambutol ocular toxicity in treatment regimens for Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2005;172(2):250–3.
Lee MS, Melamud A. Ocular Ethambutol Toxicity. In: Response. Mayo clinic proceedings: Elsevier; 2004. p. 701.
Leibold JE. The ocular toxicity of ethambutol and its relation to dose. Ann N Y Acad Sci. 1966;135(2):904–9.
Menon V, Jain D, Saxena R, et al. Prospective evaluation of visual function for early detection of ethambutol toxicity. Br J Ophthalmol. 2009;93(9):1251–4.
Roberts S. A reveiw of the papers on the ocualr toxicty of ethambutol hydrochloride, Myambutol: an anti-tuberculosis drug. Am J Optom Physiol Opt. 1974;51(12):987–92.
Smith J. Should ethambutol be barred? J Neuroophthalmol. 1987;7(2):84–6.
Tsai RK, Lee YH. Reversibility of ethambutol optic neuropathy. J Ocul Pharmacol Ther. 1997;13(5):473–7.
Yang S, Falardeau J, Winthrop K, et al. Ethambutol-induced optic neuropathy in nontuberculous mycobacterial disease. Int J Tuberc Lung Dis. 2021;25(8):680–2.
Makunyane P, Mathebula S. Update on ocular toxicity of ethambutol. Afr Vis Eye Health. 2016;75(1):1–4.
Santaella RM, Fraunfelder FW. Ocular adverse effects associated with systemic medications. Drugs. 2007;67(1):75–93.
Sadun AA, Wang MY. Ethambutol optic neuropathy: how we can prevent 100,000 new cases of blindness each year. J Neuroophthalmol. 2008;28(4):265–8.
Vistamehr S, Walsh TJ, Adelman RA. Ethambutol neuroretinopathy, Seminars in ophthalmology. Taylor & Francis. 2007. pp. 141–146.
DeVita EG, Miao M, Sadun AA. Optic neuropathy in ethambutol-treated renal tuberculosis. J Neuroophthalmol. 1987;7(2):77–86.
Matsumoto T, Kusabiraki R, Arisawa A, et al. Drastically progressive ethambutol-induced optic neuropathy after withdrawal of ethambutol: a case report and literature review. Intern Med J. 2021;60(11):1785–8.
Peets EA, Buyske DA. Comparative metabolism of ethambutol and its l-isomer. Biochem Pharmacol. 1964;13(10):1403–19.
Balhara A, Basit A, Argikar UA, Dumouchel JL, Singh S, Prasad B. Comparative proteomics analysis of the post mitochondrial supernatant fraction of human lens-free whole eye and liver. Drug Metab Dispos. 2021;49(7):592–600.
Cole A, May PM, Williams DR. Metal binding by pharmaceuticals. Part 1. Copper (II) and zinc (II) interactions following ethambutol administration. Agents Actions. 1981;11(3):296–305.
Lee CS, Brater DC, Gambertoglio JG, et al. Disposition kinetics of ethambutol in man. J Pharmacokin Biopharm. 1980;8(4):335–46.
Ladumor MK, Bhatt DK, Gaedigk A, et al. Ontogeny of hepatic sulfotransferases and prediction of age-dependent fractional contribution of sulfation in acetaminophen metabolism. Drug Metab Dispos. 2019;47(8):818–31.
Ladumor MK, Thakur A, Sharma S, et al. A repository of protein abundance data of drug metabolizing enzymes and transporters for applications in physiologically based pharmacokinetic (PBPK) modelling and simulation. Sci Rep. 2019;9(1):1–16.
Yang J, Jamei M, Yeo KR, et al. Misuse of the well-stirred model of hepatic drug clearance. Drug Metab Dispos. 2007;35(3):501–2.
Lee CS, Gambertoglio JG, Brater DC, et al. Kinetics of oral ethambutol in the normal subject. Clin Pharmacol Ther. 1977;22:615–21.
DrugBank, Ethambutol, Available from https://go.drugbank.com/salts/DBSALT000446. Accessed on 09 June 2021.
Gaohua L, Wedagedera J, Small B, et al. Development of a multi-compartment permeability‐limited lung PBPK model and its application in predicting pulmonary pharmacokinetics of anti-tuberculosis drugs. CPT: Pharmacomet Syst Pharmacol, 2015;4(10):605–613.
Becker C, Dressman J, Amidon G, et al. Biowaiver monographs for immediate release solid oral dosage forms: Ethambutol dihydrochloride. J Pharm Sci. 2008;97(4):1350–60.
Hellinen L, Hongisto H, Ramsay E, et al. Drug flux across RPE cell models: The hunt for an appropriate outer blood-retinal barrier model for use in early drug discovery. Pharmaceutics. 2020;12(2):176.
Hellinen L, Pirskanen L, Tengvall-Unadike U, et al. Retinal pigment epithelial cell line with fast differentiation and improved barrier properties. Pharmaceutics. 2019;11(8):412.
Kadam RS, Scheinman RI, Kompella UB. Pigmented-MDCK (P-MDCK) cell line with tunable melanin expression: an in vitro model for the outer blood-retinal barrier. Mol Pharm. 2012;9(11):3228–35.
Wang Q, Strab R, Kardos P, et al. Application and limitation of inhibitors in drug–transporter interactions studies. Int J Pharm. 2008;356(1–2):12–8.
Ball K, Bouzom F, Scherrmann JM, et al. Physiologically based pharmacokinetic modelling of drug penetration across the blood-brain barrier: towards a mechanistic IVIVE-based approach. AAPS J. 2013;15(4):913–32.
Del Amo EM, Rimpelä AK, Heikkinen E, et al. Pharmacokinetic aspects of retinal drug delivery. Prog Retin Eye Res. 2017;57:34–185.
Ramsay E, Hagström M, Vellonen KS, et al. Role of retinal pigment epithelium permeability in drug transfer between posterior eye segment and systemic blood circulation. Eur J Pharm Biopharm. 2019;143:18–23.
Hellinen L, Sato K, Reinisalo M, et al. Quantitative protein expression in the human retinal pigment epithelium: comparison between apical and basolateral plasma membranes with emphasis on transporters. Investig Ophthalmol Vis Sci. 2019;60(15):5022–34.
Vellonen KS, Soini EM, Del Amo EM, et al. Prediction of ocular drug distribution from systemic blood circulation. Mol Pharm. 2016;13(9):2906–11.
Vellonen KS, Hellinen L, Mannermaa E, et al. Expression, activity and pharmacokinetic impact of ocular transporters. Adv Drug Deliv Rev. 2018;126:3–22.
Saktiawati AM, Sturkenboom MG, Stienstra Y, et al. Impact of food on the pharmacokinetics of first-line anti-TB drugs in treatment-naive TB patients: a randomized cross-over trial. J Antimicrob Chemother. 2016;71(3):703–10.
Wang H, Wang R, O’Gorman M, et al. Bioequivalence of fixed-dose combination Myrin®-P Forte and reference drugs in loose combination. Int J Tuberc Lung Dis. 2013;17(12):1596–601.
Xu J, ** H, Zhu H, et al. Oral bioavailability of rifampicin, isoniazid, ethambutol, and pyrazinamide in a 4-drug fixed-dose combination compared with the separate formulations in healthy Chinese male volunteers. Clin Ther. 2013;35(2):161–8.
Strauch S, Jantratid E, Stahl M, et al. The biowaiver procedure: its application to anti-tuberculosis products in the WHO prequalification programme. J Pharm Sci. 2011;100(3):822–30.
Buyske D, Sterling W, Peets E. Pharmacological and biochemical studies on ethambutol in laboratory animals. Ann NY Acad Sci. 1966;135(2):711–25.
Chung H, Yoon YH, Hwang JJ, et al. Ethambutol-induced toxicity is mediated by zinc and lysosomal membrane permeabilization in cultured retinal cells. Toxicol Appl Pharmacol. 2009;235(2):163–70.
Delacoux E, Moreau Y, Godefroy A, et al. Prevention of ocular toxicity of ethambutol: study of zincaemia and chromatic analysis (author’s transl). J Fr Ophtalmol. 1978;1(3):191–6.
Figueroa R, Weiss H, Smith JC Jr, et al. Effect of ethambutol on the ocular zinc concentration in dogs. Am Rev Respir Dis. 1971;104(4):592–4.
Heng JE, Vorwerk CK, Lessell E, et al. Ethambutol is toxic to retinal ganglion cells via an excitotoxic pathway. Investig Ophthalmol Vis Sci. 1999;40(1):190–6.
King AB, Schwartz R. Effects of the anti-tuberculous drug ethambutol on zinc absorption, turnover and distribution in rats fed diets marginal and adequate in zinc. J Nutr. 1987;117(4):704–8.
Kozak SF, Inderlied CB, Hsu HY, et al. The role of copper on ethambutol’s antimicrobial action and implications for ethambutol-induced optic neuropathy. Diagn Microbiol Infect Dis. 1998;30(2):83–7.
Minutillo U, Miniero MA, Severino R, et al. Importance of preventing retrobulbar optic neuropathy from ethambutol by the determination of zinc in the blood and by the Farnsworth test of color sense. Monaldi Arch Chest Dis. 1980;35(4):217–28.
Shindler KS, Zurakowski D, Dreyer EB. Caspase inhibitors block zinc-chelator induced death of retinal ganglion cells. NeuroReport. 2000;11(10):2299–302.
Weismann K. Chelating drugs and zinc. Dan Med Bull. 1986;33(4):208–11.
Yoon YH, Jung KH, Sadun AA, et al. Ethambutol-induced vacuolar changes and neuronal loss in rat retinal cell culture: mediation by endogenous zinc. Toxicol Appl Pharmacol. 2000;162(2):107–14.
Chen HY, Lai SW, Muo CH, et al. Ethambutol-induced optic neuropathy: a nationwide population-based study from Taiwan. Br J Ophthalmol. 2012;96(11):1368–71.
Demiryurek BE, Gungen BD, Acar BA, et al. A case with chronic renal failure involving ethambutol and isoniazid associated bilateral optic neuritis development. Biomed Res. 2017;28(4):1500–2.
Fang JT, Chen YC, Chang MY. Ethambutol-induced optic neuritis in patients with end stage renal disease on hemodialysis: two case reports and literature review. Ren Fail. 2004;26(2):189–93.
Kanaujia V, Jain VK, Sharma K, et al. Ethambutol-induced optic neuropathy in renal disorder: a clinico-electrophysiological study. Can J Ophthalmol. 2019;54(3):301–5.
Varughese A, Brater DC, Benet LZ, et al. Ethambutol kinetics in patients with impaired renal function. Am Rev Respir Dis. 1986;134(1):34–8.
Chuenkongkaew W, Samsen P, Thanasombatsakul N. Ethambutol and optic neuropathy. J Med Assoc Thai. 2003;86(7):622–5.
Rasool M, Malik A, Manan A, et al. Determination of potential role of anti-oxidative status and circulating biochemical markers in the pathogenesis of ethambutol induced toxic optic neuropathy among diabetic and non-diabetic patients. Saudi J Biol Sci. 2015;22(6):739–43.
Chiotoroiu S, Noaghi M, Stefaniu G, et al. Tobacco-alcohol optic neuropathy-clinical challenges in diagnosis. J Med Life. 2014;7(4):472.
Acknowledgements
Authors would like to sincerely thank Simulation Plus (Lancaster, USA) for providing the academic license of Gastroplus and ADMET predictorTM to our institute, NIPER, SAS Nagar.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest statement
Authors declare no conflict of interest for the present work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Balhara, A., Ladumor, M.K., Nankar, R.P. et al. Exploration of the Plausible Mechanism of Ethambutol Induced Ocular Toxicity by Using Proteomics Informed Physiologically Based Pharmacokinetic (PBPK) Modeling. Pharm Res 39, 677–689 (2022). https://doi.org/10.1007/s11095-022-03227-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-022-03227-9