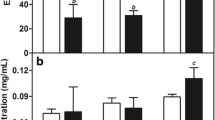
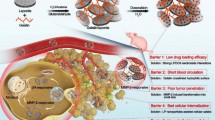
Abstract
Purpose
To develop a tumor-targeted drug delivery system based on solid lipid nanoparticles (SLNs) conjugated with the enzymatically cleavable polyethylene glycol (PEG).
Methods
SLNs loaded with paclitaxel (PTX) were prepared using the film ultrasonication method, followed by conjugation with a PEGylated peptide (Pp) that can specifically interact with matrix metalloproteinases (MMPs) that is over-expressed by tumor cells. The physicochemical characteristics of the Pp-PTX-SLNs were studied and the in vitro drug release, cytotoxicity and cell uptake of the formulations were investigated. Furthermore, using an animal model, the pharmacokinetic properties, biodistribution and anti-tumor activity of this system were evaluated.
Results
The resulting Pp-PTX-SLNs penetrated through tumor cells via facilitated uptake mediated by MMPs. The uncleavable Pp’-PTX-SLNs showed a lower cell uptake efficiency, compared with the Pp-PTX-SLNs. In a tumor-bearing mice model, Pp-PTX-SLNs accumulated to a greater extent at the tumor location, persisted longer in blood circulation, and showed lower toxicity than did PTX-SLNs or Taxol®. Most importantly, the mice treated with Pp-PTX-SLNs survived longer than the groups treated with Pp’-PTX-SLNs, PTX-SLNs or Taxol®.
Conclusions
These results suggest that Pp-PTX-SLNs hold promise as a new strategy for paclitaxel chemotherapy, and that Pp-SLNs can be a useful nanocarrier for other chemotherapeutic drugs.

Shielding the SLN with PEG2000-Gly-Pro-Leu-Gly-Ile-Ala-Gly-Gln-Cys prolongs its circulation time in blood. Cleavage of the PEG chain by tumor-secreted MMPs leads to paclitaxel uptake by target tumor cells. This SLN modification offers a new strategy for paclitaxel chemotherapy.









Similar content being viewed by others
Abbreviations
- CE:
-
Conjugation efficiency
- CHO cells:
-
Chinese hamster ovary cells
- DL%:
-
Drug loading
- EE%:
-
Encapsulation efficiency
- EPR:
-
Enhanced permeability and retention
- GMS:
-
Glyceryl monostearate
- HT1080 cells:
-
Human fibrosarcoma cells
- LLC cells:
-
Lewis lung carcinoma cells
- MCT:
-
Medium-chain triglycerides
- MMP:
-
Matrix metalloproteinase
- OA:
-
Octadecylamine
- PEG:
-
Polyethylene glycol
- Pp:
-
PEG2000-MMP substrate peptide (PEG2000-Gly-Pro-Leu-Gly-Ile-Ala-Gly-Gln-Cys)
- Pp’:
-
PEG2000-Ile-Pro-Gly-Gln-Gly-Ala-Leu-Gly-Cys
- PTX:
-
Paclitaxel
- RES:
-
Reticuloendothelial system
- SLNs:
-
Solid lipid nanoparticles
- SPC:
-
Soya phosphatidyl choline
REFERENCES
Rivkin I, Cohen K, Koffler J, Melikhov D, Peer D, Margalit R. Paclitaxel-clusters coated with hyaluronan as selective tumor-targeted nanovectors. Biomaterials. 2010;31(27):7106–14.
Guo W, Johnson JL, Khan S, Ahmad A, Ahmad I. Paclitaxel quantification in mouse plasma and tissues containing liposome-entrapped paclitaxel by liquid chromatography-tandem mass spectrometry: application to a pharmacokinetics study. Anal Biochem. 2005;336(2):213–20.
Konno T, Watanabe J, Ishihara K. Enhanced solubility of paclitaxel using water-soluble and biocompatible 2-methacryloyloxyethyl phosphorylcholine polymers. J Biomed Mater Res A. 2003;65(2):209–14.
Yoshizawa Y, Kono Y, Ogawara K, Kimura T, Higaki K. PEG liposomalization of paclitaxel improved its in vivo disposition and anti-tumor efficacy. Int J Pharm. 2011;412(1–2):132–41.
Yang T, Choi MK, Cui FD, Kim JS, Chung SJ, Shim CK, et al. Preparation and evaluation of paclitaxel-loaded PEGylated immunoliposome. J Control Release. 2007;120(3):169–77.
Hu FQ, Ren GF, Yuan H, Du YZ, Zeng S. Shell cross-linked stearic acid grafted chitosan oligosaccharide self-aggregated micelles for controlled release of paclitaxel. Colloids Surf B: Biointerfaces. 2006;50(2):97–103.
Rossi J, Giasson S, Khalid MN, Delmas P, Allen C, Leroux JC. Long-circulating poly(ethylene glycol)-coated emulsions to target solid tumors. Eur J Pharm Biopharm. 2007;67(2):329–38.
Li R, Eun JS, Lee MK. Pharmacokinetics and biodistribution of paclitaxel loaded in pegylated solid lipid nanoparticles after intravenous administration. Arch Pharm Res. 2011;34(2):331–7.
Caruthers SD, Wickline SA, Lanza GM. Nanotechnological applications in medicine. Curr Opin Biotechnol. 2007;18(1):26–30.
Kataoka K, Harada A, Nagasaki Y. Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv Drug Deliv Rev. 2001;47(1):113–31.
Mishra S, Webster P, Davis ME. PEGylation significantly affects cellular uptake and intracellular trafficking of non-viral gene delivery particles. Eur J Cell Biol. 2004;83(3):97–111.
Xu H, Deng Y, Chen D, Hong W, Lu Y, Dong X. Esterase-catalyzed dePEGylation of pH-sensitive vesicles modified with cleavable PEG-lipid derivatives. J Control Release. 2008;130(3):238–45.
Terada T, Iwai M, Kawakami S, Yamashita F, Hashida M. Novel PEG-matrix metalloproteinase-2 cleavable peptide-lipid containing galactosylated liposomes for hepatocellular carcinoma-selective targeting. J Control Release. 2006;111(3):333–42.
Kurschat P, Zigrino P, Nischt R, Breitkopf K, Steurer P, Klein CE, et al. Tissue inhibitor of matrix metalloproteinase-2 regulates matrix metalloproteinase-2 activation by modulation of membrane-type 1 matrix metalloproteinase activity in high and low invasive melanoma cell lines. J Biol Chem. 1999;274(30):21056–62.
Mansour AM, Drevs J, Esser N, Hamada FM, Badary OA, Unger C, et al. A new approach for the treatment of malignant melanoma: enhanced antitumor efficacy of an albumin-binding doxorubicin prodrug that is cleaved by matrix metalloproteinase 2. Cancer Res. 2003;63(14):4062–6.
Wan Y, Han J, Fan G, Zhang Z, Gong T, Sun X. Enzyme-responsive liposomes modified adenoviral vectors for enhanced tumor cell transduction and reduced immunogenicity. Biomaterials. 2013;34(12):3020–30.
Nikanjam M, Gibbs AR, Hunt CA, Budinger TF, Forte TM. Synthetic nano-LDL with paclitaxel oleate as a targeted drug delivery vehicle for glioblastoma multiforme. J Control Release. 2007;124(3):163–71.
Shimada K, Matsuo S, Sadzuka Y, Miyagishima A, Nozawa Y, Hirota S, et al. Determination of incorporated amounts of poly(ethylene glycol)-derivatized lipids in liposomes for the physicochemical characterization of stealth liposomes. Int J Pharm. 2000;203(1–2):255–63.
Zeng N, Hu Q, Liu Z, Gao X, Hu R, Song Q, et al. Preparation and characterization of paclitaxel-loaded DSPE-PEG-liquid crystalline nanoparticles (LCNPs) for improved bioavailability. Int J Pharm. 2012;424(1–2):58–66.
Elliott P, Hohmann A, Spanos J. Protease expression in the supernatant of Chinese hamster ovary cells grown in serum-free culture. Biotechnol Lett. 2003;25(22):1949–52.
Goutayer M, Dufort S, Josserand V, Royere A, Heinrich E, Vinet F, et al. Tumor targeting of functionalized lipid nanoparticles: assessment by in vivo fluorescence imaging. Eur J Pharm Biopharm. 2010;75(2):137–47.
Chambers AF, Matrisian LM. Changing views of the role of matrix metalloproteinases in metastasis. J Natl Cancer Inst. 1997;89(17):1260–70.
Li SD, Huang L. Stealth nanoparticles: high density but sheddable PEG is a key for tumor targeting. J Control Release. 2010;145(3):178–81.
Skubitz KM. Phase II, trial of pegylated-liposomal doxorubicin (Doxil) in sarcoma. Cancer Investig. 2003;21(2):167–76.
Kirpotin D, Hong K, Mullah N, Papahadjopoulos D, Zalipsky S. Liposomes with detachable polymer coating: destabilization and fusion of dioleoylphosphatidylethanolamine vesicles triggered by cleavage of surface-grafted poly(ethylene glycol). FEBS Lett. 1996;388(2–3):115–8.
Andreu D, Albericio F, Solé NA, Munson MC, Ferrer M, Barany G. Formation of disulfide bonds in synthetic peptides and proteins. Methods Mol Biol. 1994;35:91–169.
Drummond DC, Zignani M, Leroux J. Current status of pH-sensitive liposomes in drug delivery. Prog Lipid Res. 2000;39(5):409–60.
Jackson CJ, Arkell J, Nguyen M. Rheumatoid synovial endothelial cells secrete decreased levels of tissue inhibitor of MMP (TIMP1). Ann Rheum Dis. 1998;57(3):158–61.
Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, et al. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994;370(6484):61–5.
Allen C, Dos Santos N, Gallagher R, Chiu GN, Shu Y, Li WM, et al. Controlling the physical behavior and biological performance of liposome formulations through use of surface grafted poly(ethylene glycol). Biosci Rep. 2002;22(2):225–50.
Fang C, Shi B, Pei YY, Hong MH, Wu J, Chen HZ. In vivo tumor targeting of tumor necrosis factor-alpha-loaded stealth nanoparticles: effect of MePEG molecular weight and particle size. Eur J Pharm Sci. 2006;27(1):27–36.
Kohane DS. Microparticles and nanoparticles for drug delivery. Biotechnol Bioeng. 2007;96(2):203–9.
**ao K, Luo J, Fowler WL, Li Y, Lee JS, **ng L, et al. A self-assembling nanoparticle for paclitaxel delivery in ovarian cancer. Biomaterials. 2009;30(30):6006–16.
Martins S, Costa-Lima S, Carneiro T, Cordeiro-da-Silva A, Souto EB, Ferreira DC. Solid lipid nanoparticles as intracellular drug transporters: an investigation of the uptake mechanism and pathway. Int J Pharm. 2012;430(1–2):216–27.
Xu Z, Gu W, Huang J, Sui H, Zhou Z, Yang Y, et al. In vitro and in vivo evaluation of actively targetable nanoparticles for paclitaxel delivery. Int J Pharm. 2005;288(2):361–8.
Chen DB, Yang TZ, Lu WL, Zhang Q. In vitro and in vivo study of two types of long-circulating solid lipid nanoparticles containing paclitaxel. Chem Pharm Bull. 2001;49(11):1444–7.
Li X, Cheng X, Wang Y, Mao L, Wang Y, Huang Q. Preparation of Paclitaxel Nanosuspension and Study of Its Pharmacokinetic and Biodistrabution Behavior in Rats. Chin Pharm J. 2011;9:015.
Zhang Z, Lee SH, Gan CW, Feng SS. In vitro and in vivo investigation on PLA-TPGS nanoparticles for controlled and sustained small molecule chemotherapy. Pharm Res. 2008;25(8):1925–35.
Desai N, Trieu V, Yao Z, Louie L, Ci S, Yang A, et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin Cancer Res Off J Am Assoc Cancer Res. 2006;12(4):1317–24.
Park K. To PEGylate or not to PEGylate, that is not the question. J Control Release. 2010;142(2):147–8.
ACKNOWLEDGMENTS AND DISCLOSURES
Jie Zheng and Yu Wan contributed equally to this work. We are grateful for financial support from the University of Central Lancashire, the National Natural Science Foundation of China (No. 81173011) and the National Science & Technology Major Project of China (No. 2011ZX09401-304(4-3)).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure 1
(DOCX 87 kb)
Rights and permissions
About this article
Cite this article
Zheng, J., Wan, Y., Elhissi, A. et al. Targeted Paclitaxel Delivery to Tumors Using Cleavable PEG-Conjugated Solid Lipid Nanoparticles. Pharm Res 31, 2220–2233 (2014). https://doi.org/10.1007/s11095-014-1320-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-014-1320-8