Abstract
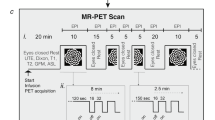
Phosphorus magnetic resonance spectroscopy (31P-MRS) combined with visual stimulation in functional experiments allows the non-invasive dynamic study of brain energy metabolism. 31P-MRS has been applied to several diseases and to healthy subjects, but works have shown variable findings and non-reproducible results, possibly caused by low numbers of subjects combined with different stimulation paradigms. In the present work, we used 31P-MRS at 3 T with two different visual stimulation protocols with different block duration (“short” and “long”) to evaluate metabolic changes under different workloads in 38 healthy subjects. We found a 15 % (short protocol—blocks of 1.5 min stimulation) and 3 % (long protocol—blocks of 5 min stimulation) increase in the inorganic phosphate (Pi) to α-adenosine triphosphate (α-ATP) ratio, and a 5 % (short protocol) and 2 % (long protocol) decrease in the nicotinamide adenine nucleotide (NADH + NAD+) to α-ATP ratio. The NADH + NAD+ results are, to the best of our knowledge, the first functional magnetic resonance spectroscopy in vivo assessment of these compounds, but their interpretation is difficult since they cannot be separately quantified at 3 T. Our results show that longer stimulations produce smaller concentration changes in Pi/α-ATP and (NADH + NAD+)/α-ATP ratios, which suggests a possible adaptation effect during longer stimulations that leads metabolic concentrations towards the initial equilibrium.



Similar content being viewed by others
References
Qiao H, Zhang X, Zhu X-H et al (2006) In vivo 31P MRS of human brain at high/ultrahigh fields: a quantitative comparison of NMR detection sensitivity and spectral resolution between 4 T and 7 T. Magn Reson Imaging 24:1281–1286
Nelson D, Cox M (2008) Lehninger principles of biochemistry, 5th edn. W.H. Freeman and Company, New York
Barker P, Butterworth E, Boska M et al (1999) Magnesium and pH imaging of the human brain at 3.0 Tesla. Magn Reson Med 41:400–406
Sappey-Marinier D, Calabrese G, Fein G et al (1992) Effect of photic stimulation on human visual cortex lactate and phosphates using 1H and 31P magnetic resonance spectroscopy. J Cereb Blood Flow Metab 12:584–592
Rango M, Castelli A, Scarlato G (1997) Energetics of 3.5 s neural activation in humans: a 31P MR spectroscopy study. Magn Reson Med 38:878–883
Murashita J, Kato T, Shioiri T et al (1999) Age-dependent alteration of metabolic response to photic stimulation in the human brain measured by 31P MR-spectroscopy. Brain Res 818:72–76
Rango M, Bonifati C, Bresolin N (2006) Parkinson’s disease and brain mitochondrial dysfunction: a functional phosphorus magnetic resonance spectroscopy study. J Cereb Blood Flow Metab 26:283–290
Murashita J, Kato T, Shioiri T et al (2000) Altered brain energy metabolism in lithium-resistant bipolar disorder detected by photic stimulated 31P-MR spectroscopy. Psychol Med 30:107–115
Rango M, Bozzali M, Prelle A et al (2001) Brain activation in normal subjects and in patients affected by mitochondrial disease without clinical central nervous system involvement: a phosphorus magnetic resonance spectroscopy study. J Cereb Blood Flow Metab 21:85–91
Kato T, Murashita J, Shioiri T et al (1998) Photic stimulation-induced alteration of brain energy metabolism measured by 31P-MR spectroscopy in patients with MELAS. J Neurol Sci 155:182–185
Mochel F, N’Guyen T, Deelchand D et al (2012) Abnormal response to cortical activation in early stages of Huntington disease. Mov Disord 27:907–910
Kato T, Murashita J, Shioiri T et al (1996) Effect of photic stimulation on energy metabolism in the human brain measured by 31P-MR spectroscopy. J Neuropsychiatry Clin Neurosci 8:417–422
Pipinos I, Shepard A, Anagnostopoulos P et al (2000) Phosphorus 31 nuclear magnetic resonance spectroscopy suggests a mitochondrial defect in claudicating skeletal muscle. J Vasc Surg 31:944–952
Longo R, Ricci C, Palma D et al (1993) Quantitative 31P MRS of the normal adult human brain. Assessment of interindividual differences and ageing effects. NMR Biomed 6:53–57
Petroff O, Prichard J, Behar K et al (1985) Cerebral intracellular pH by P-31 nuclear magnetic resonance spectroscopy. Neurology 35:781–788
Jeong E-K, Sung Y-H, Kim S-E et al (2011) Measurement of creatine kinase reaction rate in human brain using magnetization transfer image-selected in vivo spectroscopy (MT-ISIS) and a volume 31P/1H radiofrequency coil in a clinical 3-T MRI system. NMR Biomed 24:765–770
Zhu X-H, Qiao H, Du F et al (2012) Quantitative imaging of energy expenditure in human brain. Neuroimage 60:2107–2117
Chen W, Zhu X, Adriany G, Ugurbil K (1997) Increase of creatine kinase activity in the visual cortex of human brain during visual stimulation: a 31P magnetization transfer study. Magn Reson Med 38:551–557
Cadoux-Hudson T, Blackledge M, Radda G (1989) Imaging of human brain creatine kinase activity in vivo. FASEB J 3:2660–2666
Vidyasagar R, Kauppinen R (2008) 31P magnetic resonance spectroscopy study of the human visual cortex during stimulation in mild hypoxic hypoxia. Exp Brain Res 187:229–235
Chen C, Gowland P, Francis S et al (2014) Assessing activation induced changes in creatine kinase activity in the human brain using 31P spectroscopy with magnetization transfer. In: Proceedings 22nd scientific meeting and exhibition international society for magnetic resonance in medicine 462
Lee B, Zhu X-H, Chen W (2014) Elevated ATP synthase and creatine kinase activities in human visual cortex during visual stimulation: a 31P NMR magnetization transfer study at 7 T. In: Proceedings 22nd scientific meeting and exhibition international society for magnetic resonance in medicine 0012
Lin A-L, Fox PT, Hardies J et al (2010) Nonlinear coupling between cerebral blood flow, oxygen consumption, and ATP production in human visual cortex. Proc Natl Acad Sci USA 107:8446–8451
Frahm J, Krüger G, Merboldt K, Kleinschmidt A (1996) Dynamic uncoupling and recoupling of perfusion and oxidative metabolism during focal brain activation in man. Magn Reson Med 35:143–148
Kasischke K, Vishwasrao H, Fisher P et al (2004) Neural activity triggers neuronal oxidative metabolism followed by astrocytic glycolysis. Science 305(5680):99–103
Hall C, Klein-Flugge M, Howarth C, Attwell D (2012) Oxidative phosphorylation, not glycolisys, powers presynaptic and postsynaptic mechanisms underlying brain information processing. J Neurosci 32:8940–8951
Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A (2001) Neurophysiological investigation of the basis of the fMRI signal. Nature 412(6843):150–157
Acknowledgments
This study was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, Brazil—Grants 2005/56578-4, 2009/00270-2, 2009/10046-2, 2011/01106-1) and Conselho Nacional de Pesquisa (CNPq, Brazil—Grant 500148/2011-2).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barreto, F.R., Costa, T.B.S., Landim, R.C.G. et al. 31P-MRS Using Visual Stimulation Protocols with Different Durations in Healthy Young Adult Subjects. Neurochem Res 39, 2343–2350 (2014). https://doi.org/10.1007/s11064-014-1433-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-014-1433-9