Abstract
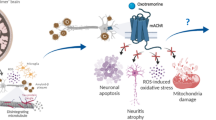
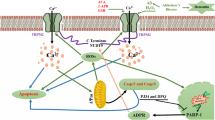
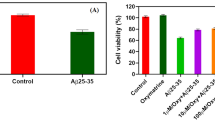
The aggregation and accumulation of amyloid-β (Aβ) plays a significant role in the pathogenesis of Alzheimer’s disease. Aβ is known to increase free radical production in neuronal cells, leading to oxidative stress and cell death. Diazoxide (DZ), a highly selective drug capable of opening mitochondrial ATP-sensitive potassium channels, has neuroprotective effects against neuronal cell death. However, the mechanism through which DZ protects cholinergic neurons against Aβ-induced oxidative injury is still unclear. The present study was designed to investigate the effects of DZ pretreatment against Aβ1–42 induced oxidative damage and cytotoxicity. Through measures of DZ effects on Aβ1–42 induced cellular damage, reactive oxygen species (ROS) and MDA generation and expressions of gp91phox and p47phox in cholinergic neurons, new insights into the neuroprotective mechanisms can be derived. Aβ1–42 significantly decreased 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide levels and increased ROS and MDA production; all effects were attenuated by pretreatment with DZ or diphenyleneiodonium chloride (a NOX2 inhibitor). Pretreatment with DZ also attenuated the upregulation of NOX2 subunits (gp91phox and p47phox) induced by Aβ1–42. Since NOX2 is one of the main sources of free radicals, these results suggest that DZ can counteract Aβ1–42 induced oxidative stress and associated cell death by reducing the level of ROS and MDA, in part, by alleviating NOX2 expression.






Similar content being viewed by others
References
Sultana R, Butterfield DA (2010) Role of oxidative stress in the progression of Alzheimer’s disease. J Alzheimers Dis 19:341–353
Murphy MP (2009) How mitochondria produce reactive oxygen species. Biochem J 417:1–13
Sheng B, Wang X, Su B et al (2012) Impaired mitochondrial biogenesis contributes to mitochondrial dysfunction in Alzheimer’s disease. J Neurochem 120:419–429
Wang X, Su B, Lee HG et al (2009) Impaired balance of mitochondrial fission and fusion in Alzheimer’s disease. J Neurosci 29:9090–9103
Zhu **aolei, Chen Cong, Ye Dan et al (2012) Diammonium glycyrrhizinate upregulates PGC-1a and protects against Ab1–42-induced neurotoxicity. PLoS ONE 7:e35823
Petrozzi L, Ricci G, Giglioli N et al (2007) Mitochondria and neurodegeneration. Biosci Rep 27:87–104
Correia Sónia C, Carvalho Cristina et al (2010) Mitochondrial preconditioning: a potential neuroprotective Strategy. Front Aging Neurosci 2:138
Chen Shan, Meng **an-Fang, Zhang Chun (2013) Role of NADPH oxidase-mediated reactive oxygen species in podocyte injury. Biomed Res Int 2013:839761
Gao Hui-Ming, Zhou Hui, Hong Jau-Shyong (2012) NADPH oxidases: novel therapeutic targets for neurodegenerative diseases. Trends Pharmacol Sci 33:295–303
Ansari MA, Scheff SW (2011) NADPH-oxidase activation and cognition in Alzheimer disease progression. Free Radical Biol Med 51:171–178
Shimohama S (2011) Activation of NADPH oxidase in Alzheimer’s disease brains. Biochem Biophys Res Commun 273:5–9
Virgili Noe, Mancera Pilar, Wappenhans Blanca et al (2013) KATP channel opener diazoxide prevents neurodegeneration: a new mechanism of action via antioxidative pathway activation. PLoS ONE 8:e75189
Busija DW, Gaspar T, Domoki F et al (2008) Mitochondrialmediated suppression of ROS production upon exposure of neurons to lethal stress: mitochondrial targeted preconditioning. Adv Drug Deliv Rev 60:1471–1477
**e J, Duan L, Qian X et al (2010) K(ATP) channel openers protect mesencephalic neurons against MPP + -induced cytotoxicity via inhibition of ROS production. J Neurosci Res 88:428–437
Takashi E, Wang Y, Ashraf M (1999) Activation of mitochondrial K(ATP) channel elicits late preconditioning against myocardial infarction via protein kinase C signaling pathway. Circ Res 85:1113–1114
Liu D, Lu C, Wan R et al (2002) Activation of mitochondrial ATP-dependent potassium channels protects neurons against ischemia-induced death by a mechanism involving suppression of Bax translocation and cytochrome c release. J Cereb Blood Flow Metab 22:431–443
Zeng X, Wang T, Jiang L et al (2013) Diazoxide and cyclosporin A protect primary cholinergic neurons against beta-amyloid (1-42)-induced cytotoxicity. Neurol Res 35:529–536
Ma G, Fu Q, Zhang Y et al (2008) Effects of Abeta1-42 on the subunits of KATP expression in cultured primary rat basal forebrain neurons. Neurochem Res 33:1419–1424
Ma G, Gao J, Fu Q et al (2009) Diazoxide reverses the enhanced expression of KATP subunits in cholinergic neurons caused by exposure to Aβ1–42. Neurochem Res 34:2133–2140
Gao HM, Zhou H, Hong JS (2012) NADPH oxidases: novel therapeutic targets for neurodegenerative diseases. Trends Pharmacol Sci 33:295–303
Kadowaki H, Nishitoh H, Urano F et al (2005) Amyloid beta induces neuronal cell death through ROS-mediated ASK1 activation. Cell Death Differ 12:19–24
Muthaiyah B, Essa MM, Chauhan V et al (2011) Protective effects of walnut extract against amyloid beta peptide-induced cell death and oxidative stress in PC12cells. Neurochem Res 36:2096–2103
Sponne I, Fifre A, Drouet B et al (2003) Apoptotic neuronal cell death induced by the non-fibrillar amyloid-beta peptide proceeds through an early reactive oxygen species-dependent cytoskeleton perturbation. J Biol Chem 278:3437–3445
Goodman Y, Mattson MP (1996) K+ channel openers protect hippocampal neurons against oxidative injury and amyloid beta-peptide toxicity. Brain Res 706:328–332
Gao HM, Hong JS (2008) Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Trends Immunol 29:357–365
Philips T, Robberecht W (2011) Neuroinflammation in amyotrophic lateral sclerosis: role of glial activation in motor neuron disease. Lancet Neurol 10:253–263
Glass CK (2010) Mechanisms underlying inflammation in neurodegeneration. Cell 140:918–934
Coyoy A (2008) Role of NADPH oxidase in the apoptotic death of cultured cerebellar granule neurons. Free Radical Biol Med 45:1056–1064
Guemez-Gamboa A, Moran J (2009) NOX2 mediates apoptotic death induced by staurosporine but not by potassium deprivation in cerebellar granule neurons. J Neurosci Res 87:2531–2540
Abramov AY (2004) Beta-amyloid peptides induce mitochondrial dysfunction and oxidative stress in astrocytes and death of neurons through activation of NADPH oxidase. J Neurosci 24:565–575
Park L (2008) Nox2-derived radicals contribute to neurovascular and behavioral dysfunction in mice overexpressing the amyloid precursor protein. Proc Natl Acad Sci USA 105:1347–1352
Baines CP, Cohen MV, Downey JM (1999) Signal transduction in ischemic preconditioning: the role of kinases and mitochondrial K(ATP) channels. J Cardiovasc Electrophysiol 10:741–754
Tai KK, McCrossan ZA, Abbott GW (2003) Activation of mitochondrial ATP-sensitive potassium channels increases cell viability against rotenone-induced cell death. J Neurochem 84:1193–1200
Teshima Y, Akao M, Li RA (2003) MitoKATP channel activation protects cerebellar granule neurons from apoptosis induced by oxidative stress. Stroke 34:1796–1802
Dikalov S (2011) Cross talk between mitochondria and NADPH oxidases. Free Radic Biol Med 51:1289–1301
Rey FE, Cifuentes ME, Kiarash A et al (2001) Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O(2)(−) and systolic blood pressure in mice. Circ Res 89:408–414
Lee SB, Bae IH, Bae YS et al (2006) Link between mitochondria and NADPH oxidase 1 isozyme for the sustained production of reactive oxygen species and cell death. J Biol Chem 281:36228–36235
Rathore R, Zheng YM, Niu CF et al (2008) Hypoxia activates NADPH oxidase to increase [ROS]i and [Ca2+]i through the mitochondrial ROS-PKCepsilon signaling axis in pulmonary artery smooth muscle cells. Free Radic Biol Med 45:1223–1231
Irrcher I, Ljubicic V, Hood DA (2009) Interactions between ROS and AMP kinase activity in the regulation of PGC-1alpha transcription in skeletal muscle cells. Am J Physiol Cell Physiol 296:C116–C123
Kim HJ, Park KG, Yoo EK et al (2007) Effects of PGC-1alpha on TNF-alpha-induced MCP-1 and VCAM-1 expression and NF-kappaB activation in human aortic smooth muscle and endothelial cells. Antioxid Redox Signal 9:301–307
Lee C, Miura K, Liu X et al (2000) Biphasic regulation of leukocyte superoxide generation by nitric oxide and peroxynitrite. J Biol Chem 275:38965–38972
Carey RM (2005) Update on the role of the AT2 receptor. Curr Opin Nephrol Hypertens 14:67–71
Dikalova AE, Bikineyeva AT, Budzyn K et al (2010) Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res 107:106–116
Ballinger SW (2005) Mitochondrial dysfunction in cardiovascular disease. Free Radic Biol Med 38:1278–1295
Wang L, Zhu QL, Wang GZ et al (2011) The protective roles of mitochondrial ATP-sensitive potassium channels during hypoxiaischemia-reperfusion in brain. Neurosci Lett 491:63–67
Correia SC, Santos RX, Perry G et al (2010) Mitochondria: the missing link between preconditioning and neuroprotection. J Alzheimers Dis 20(Suppl 2):S475–S485
Acknowledgments
This work was supported by the Young Scholars General Program of the National Natural Science Foundation (China, No. 30600202); the International Exchange and Cooperation Program of National Natural Science Foundation (China, No. 30710303072); and the General Program of the National Natural Science Foundation (China, No. 30870874).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Fu, Q., Gao, N., Yu, J. et al. Diazoxide Pretreatment Prevents Aβ1–42 Induced Oxidative Stress in Cholinergic Neurons Via Alleviating NOX2 Expression. Neurochem Res 39, 1313–1321 (2014). https://doi.org/10.1007/s11064-014-1313-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-014-1313-3