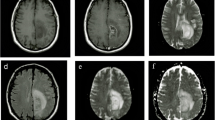
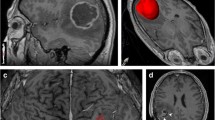
Abstract
Treatment for brain gliomas is a combined approach of surgery, radiation therapy and chemotherapy. Nevertheless, high-grade gliomas usually recur despite treatment. Ionizing radiation therapy to the central nervous system may cause post-radiation damage. Differentiation between post-irradiation necrosis and recurrent glioma on the basis of clinical signs and symptomatology has not been possible. Computed tomography (CT) and magnetic resonance imaging (MRI) suffer from significant limitations when applied to differentiate recurrent brain tumor from radiation necrosis. We reviewed the contribution of recent MRI techniques, single-photon emission CT and positron emission tomography to discriminate necrosis for glioma recurrence. We concluded that despite the progress being made, further research is needed to establish reliable imaging modalities that distinguish between true tumour progression and treatment-related necrosis.
Similar content being viewed by others
References
Glass JP, Hwang T-L, Leavens ME, Libshitz HI (1984) Cerebral radiation necrosis following treatment of extracranial malignancies. Cancer 54:1966–1972. doi:10.1002/1097-0142(19841101)54:9<1966::AID-CNCR2820540930>3.0.CO;2-4
Fischer AW, Holfelder H (1930) Lokales amyloid im gehirn. Dtsch Z Chir 227:475–483
Marks JE, Wong J (1985) The risk of cerebral radionecrosis in relation to dose, time and fractionation: a followup study. Prog Exp Tumor Res 29:210–218
Yoshii Y (2008) Pathological review of late cerebral radionecrosis. Brain Tumor Pathol 25:51–58. doi:10.1007/s10014-008-0233-9
DeAngelis LM (2001) Brain tumors. N Engl J Med 344:114–123. doi:10.1056/NEJM200101113440207
Brandsma D, Stalpers L, Taal W et al (2008) Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol 9:453–461. doi:10.1016/S1470-2045(08)70125-6
Brandes AA, Franceschi E, Tosoni A et al (2008) MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol 1(26):2192–2197. doi:10.1200/JCO.2007.14.8163
Chamberlain MC, Glantz MJ, Chalmers L et al (2007) Early necrosis following concurrent Temodar and radiotherapy in patients with glioblastoma. J Neurooncol 82:81–83. doi:10.1007/s11060-006-9241-y
Burger PC (1986) Malignant astrocytic neoplasms: classification, pathologic anatomy, and response to treatment. Semin Oncol 13:16–26
Perry A, Schmidt RE (2006) Cancer therapy-associated CNS neuropathology: an update and review of the literature. Acta Neuropathol 111:197–212. doi:10.1007/s00401-005-0023-y
Burger PC, Scheithauer BW (1994) Atlas of tumor pathology, 3rd series, fascicle 10: tumors of the central nervous system. Armed Forces Institute of Pathology, Washington, DC
Carvalho PA, Schwartz RB, Alexander E III (1992) Detection of recurrent gliomas with quantitative thallium-201/technetium-99m HMPAO single-photon emission computerized tomography. J Neurosurg 77:565–570
Byrne TN (1994) Imaging of gliomas. Semin Oncol 21:162–171
Leeds NE, Jackson EF (1994) Current imaging techniques for the evaluation of brain Neoplasms. Curr Opin Oncol 6:254–261. doi:10.1097/00001622-199405000-00006
Kumar AJ, Leeds NE, Fuller GN (2000) Malignant gliomas: MR imaging spectrum of radiation therapy- and hemotherapy-induced necrosis of the brain after treatment. Radiology 217:377–384
Mullins ME, Barest GD, Schaefer PW, Hochberg FH, Gonzalez RG, Lev MH (2005) Radiation necrosis versus glioma recurrence: conventional MR imaging clues to diagnosis. AJNR Am J Neuroradiol 26:1967–1972
Stadnik TW, Chaskis C, Michotte A (2001) Diffusion-weighted MR imaging of intracerebral masses: comparison with conventional MR imaging and histologic findings. AJNR Am J Neuroradiol 22:969–976
Schaefer PW, Ozsunar Y, He J (2003) Assessing tissue viability with MR diffusion and perfusion imaging. AJNR Am J Neuroradiol 24:436–443
Marks JE, Baglan RJ, Prassad SC, Blank WF (1981) Cerebral radionecrosis: incidence and risk in relation to dose, time, fractionation and volume. Int J Radiat Oncol Biol Phys 7:243–252
Brunberg JA, Chenevert TL, McKeever PE (1995) In vivo MR determination of water diffusion coefficients and diffusion anisotropy: correlation with structural alteration in gliomas of the cerebral hemispheres. AJNR Am J Neuroradiol 16:361–371
Lu S, Ahn D, Johnson G (2003) Peritumoral diffusion tensor imaging of high grade gliomas and metastatic brain tumors. AJNR Am J Neuroradiol 24:937–941
Lu S, Ahn D, Johnson G et al (2004) Diffusion-tensor MR imaging of intracranial neoplasia and associated peritumoral edema: introduction of the tumor infiltration index. Radiology 232:221–228. doi:10.1148/radiol.2321030653
Oppenheim C, Rodrigo S, Poupon C et al (2004) Diffusion tensor MR imaging of the brain: clinical applications. J Radiol 85:287–296 in French
Le Bihan D, Douek P, Argyropoulou M et al (1993) Diffusion and perfusion magnetic resonance imaging in brain tumors. Top Magn Reson Imaging 5:25–31
Castillo M, Smith JK, Kwock L, Wilber K (2001) Apparent diffusion coefficients in the evaluation of high-grade cerebral gliomas. AJNR Am J Neuroradiol 22:60–64
Kitis O, Altay H, Calli C et al (2005) Minimum apparent diffusion coefficients in the evaluation of brain tumors. Eur J Radiol 55:393–400. doi:10.1016/j.ejrad.2005.02.004
Kono K, Inoue Y, Nakayama K et al (2001) The role of diffusion-weighted imaging in patients with brain tumors. AJNR Am J Neuroradiol 22:1081–1088
Lam WW, Poon WS, Metreweli C (2002) Diffusion MR imaging in glioma: does it have any role in the pre-operation determination of grading of glioma? Clin Radiol 57:219–225. doi:10.1053/crad.2001.0741
Asao C, Korogi Y, Kitajima M et al (2005) Diffusion-weighted imaging of radiation-induced brain injury for differentiation from tumor recurrence. AJNR Am J Neuroradiol 26:1455–1460
Hein PA, Eskey CJ, Dunn JF, Hug EB (2004) Diffusion-weighted imaging in the follow-up of treated high-grade gliomas: tumor recurrence versus radiation injury. AJNR Am J Neuroradiol 25:201–209
Biousse V, Newman NJ, Hunter SB, Hudgins PA (2003) Diffusion weighted imaging in radiation necrosis. J Neurol Neurosurg Psychiatry 74:382–384. doi:10.1136/jnnp.74.3.382
Sundgren PC, Fan X, Weybright P et al (2006) Differentiation of recurrent brain tumor versus radiation injury using diffusion tensor imaging in patients with new contrast-enhancing lesions. Magn Reson Imaging 24:1131–1142. doi:10.1016/j.mri.2006.07.008
Kashimura H, Inoue T, Beppu T et al (2007) Diffusion tensor imaging for differentiation of recurrent brain tumor and radiation necrosis after radiotherapy—three case reports. Clin Neurol Neurosurg 109:106–110. doi:10.1016/j.clineuro.2006.04.005
Covarrubias DJ, Rosen BR, Lev MH (2004) Dynamic magnetic resonance perfusion imaging of brain tumors. Oncologist 9:528–537. doi:10.1634/theoncologist.9-5-528
Aronen HJ, Perkiö J (2002) Dynamic susceptibility contrast MRI of gliomas. Neuroimaging Clin N Am 12:501–523. doi:10.1016/S1052-5149(02)00026-6
Sugahara T, Korogi Y, Tomiguchi S et al (2000) Posttherapeutic intraaxial brain tumor: the value of perfusion-sensitive contrast- enhanced MR imaging for differentiating tumor recurrence from nonneoplastic contrast-enhancing tissue. AJNR Am J Neuroradiol 21:901–909
Di Costanzo A, Pollice S, Trojsi F et al (2008) Role of perfusion-weighted imaging at 3 Tesla in the assessment of malignancy of cerebral gliomas. Radiol Med 113:134–143. doi:10.1007/s11547-008-0232-2 (Torino)
Hollingworth LS, Medina RE, Lenkinski DK et al (2006) A systematic literature review of magnetic resonance spectroscopy for the characterization of brain tumors. AJNR Am J Neuroradiol 27:1404–1411
Ando K, Ishikura R, Nagami Y et al (2004) Usefulness of Cho/Cr ratio in protonMR spectroscopy for differentiating residual/recurrent glioma from non-neoplastic lesions. Nippon Igaku Hoshasen Gakkai Zasshi 64:121–126
Traber F, Block W, Flacke S et al (2002) 1H-MR Spectroscopy of brain tumors in the course of radiation therapy: use of fast spectroscopic imaging and singlevoxel spectroscopy for diagnosing recurrence. Rofo 174:33–42
Lichy MP, Henze M, Plathow C et al (2004) Metabolic imaging to follow stereotactic radiation of gliomas—the role of1HMRspectroscopy in comparison toFDGPET and IMT-SPECT. Rofo 176:1114–1121
Plotkin M, Eisenacher J, Bruhn H et al (2004) 123I-IMT SPECT and1HMR-spectroscopy at 3.0 T in the differential diagnosis of recurrent or residual gliomas: a comparative study. J Neurooncol 70:49–58. doi:10.1023/B:NEON.0000040810.77270.68
Dowling C, Bollen AW, Noworolski SM et al (2001) Preoperative proton MR spectroscopic imaging of brain tumors: correlation with histopathologic analysis of resection specimens. AJNR Am J Neuroradiol 22:604–612
Zeng QS, Li CF, Liu H (2007) Distinction between recurrent glioma and radiation injury using magnetic resonance spectroscopy in combination with diffusion-weighted imaging. Int J Radiat Oncol Biol Phys 68:151–158. doi:10.1016/j.ijrobp.2006.12.001
Rock JP, Scarpace L, Hearshen D et al (2004) Associations among magnetic resonance spectroscopy, apparent diffusion coefficients, and image-guided histopathology with special attention to radiation necrosis. Neurosurgery 54:1111–1117. doi:10.1227/01.NEU.0000119328.56431.A7
Chen W (2007) Clinical applications of PET in brain tumors. J Nucl Med 48:1468–1481. doi:10.2967/jnumed.106.037689
Bénard F, Romsa J, Hustinx R (2002) Imaging gliomas with positron emission tomography and single-photon emission computed tomography. Semin Nucl Med 33:148–162. doi:10.1053/snuc.2003.127304
Vos MJ, Tony BN, Hoekstra OS et al (2007) Systematic review of the diagnostic accuracy of 201Tl single photon emission computed tomography in the detection of recurrent glioma. Nucl Med Commun 28:431–439. doi:10.1097/MNM.0b013e328155d131
Kline JL, Noto RB, Glantz M (1996) Single-photon emission CT in the evaluation of recurrent brain tumor in patients treated with gamma knife radiosurgery or conventional radiation therapy. AJNR Am J Neuroradiol 17:1681–1686
Le Jeune FP, Dubois F, Blond S et al (2006) Sestamibi technetium-99m brain single-photon emission computed tomography to identify recurrent glioma in adults: 201 studies. J Neurooncol 77:177–183. doi:10.1007/s11060-005-9018-8
Henze M, Mohammed A, Schlemmer HP et al (2004) PET and SPECT for detection of tumor progression in irradiated low-grade astrocytoma: a receiver-operating-characteristic analysis. J Nucl Med 45:579–586
Yamamoto Y, Nishiyama Y, Toyama Y et al (2002) 99mTc-MIBI and 201Tl SPET in the detection of recurrent brain tumours after radiation therapy. Nucl Med Commun 23:1183–1190. doi:10.1097/00006231-200212000-00006
Lamy-Lhullier C, Dubois F, Blond S et al (1999) Importance of cerebral tomoscintigraphy using technetium-labeled sestamibi in the differential diagnosis of current tumor vs radiation necrosis in subtentorial glial tumors in the adult. Neurochirurgie 45:110–117
Barai S, Bandopadhayaya GP, Julka PK et al (2004) Evaluation of 99mTc-L-methionine brain SPECT for detection of recurrent brain tumor: a pilot study with radiological and pathological correlation. Acta Radiol 45:649–657. doi:10.1080/02841850410006740
Schwartz RB, Carvalho PA, Alexander E III et al (1991) Radiation necrosis vs high-grade recurrent glioma: differentiation by using dual-isotope SPECT with 201TI and 99mTc-HMPAO. AJNR Am J Neuroradiol 12:1187–1192
Slizofski WJ, Krishna L, Katsetos CD et al (1994) Thallium imaging for brain tumors with results measured by a semiquantitative index and correlated with histopathology. Cancer 74:3190–3197. doi:10.1002/1097-0142(19941215)74:12%3c3190::AID-CNCR2820741218%3e3.0.CO;2-#
Barai S, Bandopadhayaya G, Julka P et al (2004) Imaging of recurrent brain tumors with trivalent (99m)Tc-dimercaptosuccinic acid-initial results. Hell J Nucl Med 7:44–47
Barai S, Rajkamal, Bandopadhayaya GP et al (2005) Thallium-201 versus Tc99m-glucoheptonate SPECT for evaluation of recurrent brain tumours: a within-subject comparison with pathological correlation. J Clin Neurosci 12:27–31. doi:10.1016/j.jocn.2004.01.008
Barai S, Bandopadhayaya GP, Julka PK et al (2004) Role of Tc-glucoheptonic acid brain single photon emission computed tomography in differentiation of recurrent brain tumour and post-radiation gliosis. Australas Radiol 48:296–301. doi:10.1111/j.0004-8461.2004.01310.x
Tsiouris S, Pirmettis I, Chatzipanagiotou T et al (2007) Pentavalent technetium-99m dimercaptosuccinic acid [99m Tc-(V)DMSA] brain scintitomography—a plausible non-invasive depicter of glioblastoma proliferation and therapy response. J Neurooncol 85:291–295. doi:10.1007/s11060-007-9410-7
Alexiou GA, Fotopoulos AD, Papadopoulos A et al (2007) Evaluation of brain tumor recurrence by (99m)Tc-tetrofosmin SPECT: a prospective pilot study. Ann Nucl Med 21:293–298. doi:10.1007/s12149-007-0027-x
Barai S, Bandopadhayaya GP, Julka PK et al (2003) Evaluation of single photon emission computerised tomography (SPECT) using Tc99m-tetrofosmin as a diagnostic modality for recurrent posterior fossa tumours. J Postgrad Med 49:316–320
Yoshii Y, Satou M, Yamamoto T et al (1993) The role of thallium-201 single photon emission tomography in the investigation and characterisation of brain tumours in man and their response to treatment. Eur J Nucl Med 20:39–45. doi:10.1007/BF02261244
Macapinlac H, Scott A, Caluser C et al (1992) Comparison of T1-201 and Tc-99m-2-methoxy isobutyl isonitrile (MIBI) with MRI in the evaluation of recurl-ent brain tumors. J Nucl Med 33:867
Kosuda S, Fujii H, Aoki S et al (1993) Reassessment of quantitative thallium-201 brain SPECT for miscellaneous brain tumors. Ann Nucl Med 7:257–263
Sasaki M, Ichiya Y, Kuwabara Y et al (1996) Hyperperfusion and hypermetabolism in brain radiation necrosis with epileptic activity. J Nucl Med 37:1174–1176
Yoshii Y, Moritake T, Suzuki K et al (1996) Cerebral radiation necrosis with accumulation of thallium 201 on single-photon emission CT. AJNR Am J Neuroradiol 17:1773–1776
Soler C, Beauchesne P, Maatougui K et al (1998) Technetium-99m sestamibi brain single-photon emission tomography for detection of recurrent gliomas after radiation therapy. Eur J Nucl Med 25:1649–1657. doi:10.1007/s002590050344
Mountz JM, Rosenfeld SS, Li Y (1993) Utility of T1-201 and Tc-99m-sestamibi SPECT for early determination of malignant tumor chemotherapy efficacy. J NucI Med 34:206R
Borodin OYU, Velichko OB, Garganeev AB, et al. (2000) Comparison of 99 mTc-MIBI SPECT and GD-enhanced MRI in detection of recurrent tumor in malignant. Eur J Nucl Med 27:154R
Palumbo B, Lupattelli M, Pelliccioli GP et al (2006) Association of 99mTc-MIBI brain SPECT and proton magnetic resonance spectroscopy (1H-MRS) to assess glioma recurrence after radiotherapy. Q J Nucl Med Mol Imaging 50:88–93
Alexiou GA, Tsiouris S, Goussia A et al (2008) Evaluation of glioma proliferation by 99mTc-Tetrofosmin. Neurooncology 10:104–105. doi:10.1215/15228517-2007-043
Alexiou GA, Vartholomatos G, Tsiouris S et al (2008) Evaluation of meningioma aggressiveness by (99m)Tc-Tetrofosmin SPECT. Clin Neurol Neurosurg 110:645–648. doi:10.1016/j.clineuro.2008.03.016
Fotopoulos AD, Alexiou GA, Goussia A et al (2008) (99m)Tc-Tetrofosmin brain SPECT in the assessment of meningiomas-correlation with histological grade and proliferation index. J Neurooncol 89:225–230. doi:10.1007/s11060-008-9611-8
Barai S, Bandopadhayaya GP, Julka PK et al (2004) Imaging using Tc99m-tetrofosmin for the detection of the recurrence of brain tumour: a comparative study with Tc99m-glucoheptonate. J Postgrad Med 50:89–93
Samnick S, Bader JB, Hellwig D et al (2002) Clinical value of iodine-123-alpha-methyl-l-tyrosine single-photon emission tomography in the differential diagnosis of recurrent brain tumor in patients pretreated for glioma at follow-up. J Clin Oncol 20:396–404. doi:10.1200/JCO.20.2.396
Kuwert T, Woesler B, Morgenroth C et al (1998) Diagnosis of recurrent glioma with SPECT and iodine-123-alpha-methyl tyrosine. J Nucl Med 39:23–27
Henze M, Mohammed A, Schlemmer H et al (2002) Detection of tumour progression in the follow-up of irradiated low-grade astrocytomas: comparison of 3-[123I]iodo-alpha-methyl-l-tyrosine and 99mTc-MIBI SPET. Eur J Nucl Med Mol Imaging 29:1455–1461. doi:10.1007/s00259-002-0896-0
Di Chiro G, Oldfield E, Wright DC et al (1988) Cerebral necrosis after radiotherapy and/or intraarterial chemotherapy for brain tumors: PET and neuropathologic studies. AJR Am J Roentgenol 150:189–197
Doyle WK, Budinger TF, Valk PE et al (1987) Differentiation of cerebral radiation necrosis from tumor recurrence by [18F]FDG and 82Rb positron emission tomography. J Comput Assist Tomogr 11:563–570. doi:10.1097/00004728-198707000-00001
Kim EE, Chung S-K, Haynie TP et al (1992) Differentiation of residual or recurrent tumors from post-treatment changes in F-18 FDG PET. Radiographics 12:269–279
Valk PE, Budinger TF, Levin VA (1988) PET of malignant tumors after interstitial brachytherapy: demonstration of metabolic activity and correlation with clinical outcome. J Neurosurg 69:830–838
Glantz MJ, Hoffman JM, Coleman RE et al (1991) Identification of early recurrence of primary central nervous system tumors by 18F fluorodeoxyglucose positron emission tomography. Ann Neurol 29:347–355. doi:10.1002/ana.410290403
Ogawa T, Kanno I, Shishido F et al (1991) Clinical value of PET with [18F]fluorodeoxyglucose and l-methyl-11C-methionine for diagnosis of recurrent brain tumor and radiation injury. Acta Radiol 32:197–202
Ricci PE, Karis JP, Heiserman JE et al (1998) Differentiating recurrent tumor from radiation necrosis: time for re-evaluation of positron emission tomography? AJNR Am J Neuroradiol 19:407–413
Olivero WC, Dulebohn SC, Lister JR (1995) The use of PET in evaluating patients with primary brain tumors: is it useful? J Neurol Neurosurg Psychiatry 58:250–252. doi:10.1136/jnnp.58.2.250
Gómez-Río M, Rodríguez-Fernández A, Ramos-Font C et al (2008) Diagnostic accuracy of 201Thallium-SPECT and 18F-FDG-PET in the clinical assessment of glioma recurrence. Eur J Nucl Med Mol Imaging 35:966–975. doi:10.1007/s00259-007-0661-5
Kahn D, Follett KA, Bushnell DL et al (1994) Diagnosis of recurrent brain tumor: value of 201Tl SPECT vs 18F-fluorodeoxyglucose PET. AJR Am J Roentgenol 163:1459–1465
Stokkel M, Stevens H, Taphoorn M et al (1999) Differentiation between recurrent brain tumour and post-radiation necrosis: the value of 201Tl SPET versus 18F-FDG PET using a dual-headed coincidence camera—a pilot study. Nucl Med Commun 20:411–417
Terakawa Y, Tsuyuguchi N, Iwai Y et al (2008) Diagnostic accuracy of 11C-methionine PET for differentiation of recurrent brain tumors from radiation necrosis after radiotherapy. J Nucl Med 49:694–699. doi:10.2967/jnumed.107.048082
Tsuyuguchi N, Takami T, Sunada I et al (2004) Methionine positron emission tomography for differentiation of recurrent brain tumor and radiation necrosis after stereotactic radiosurgery—in malignant glioma. Ann Nucl Med 18:291–296. doi:10.1007/BF02984466
Sonoda Y, Kumabe T, Takahashi T et al (1998) Clinical usefulness of 11C-MET PET and 201T1 SPECT for differentiation of recurrent glioma from radiation necrosis. Neurol Med Chir 38:342–347. doi:10.2176/nmc.38.342 (Tokyo)
Van Laere K, Ceyssens S, Van Calenbergh F et al (2005) Direct comparison of 18F-FDG and 11C-methionine PET in suspected recurrence of glioma: sensitivity, inter-observer variability and prognostic value. Eur J Nucl Med Mol Imaging 32:39–51. doi:10.1007/s00259-004-1564-3
Jacobs AH, Thomas A, Kracht LW et al (2005) 18F-fluoro-l-thymidine and 11C-methylmethionine as markers of increased transport and proliferation in brain tumors. J Nucl Med 46:1948–1958
Chen W, Cloughesy T, Kamdar N et al (2005) Imaging proliferation in brain tumors with 18F-FLT PET: comparison with 18F-FDG. J Nucl Med 46:945–952
Yamamoto Y, Wong TZ, Turkington TG et al (2006) 3′-Deoxy-3′-[F-18]fluorothymidine positron emission tomography in patients with recurrent glioblastoma multiforme: comparison with Gd-DTPA enhanced magnetic resonance imaging. Mol Imaging Biol 8:340–347. doi:10.1007/s11307-006-0063-2
Chen W, Silverman DH, Delaloye S et al (2006) 18F-FDOPA PET imaging of brain tumors: comparison study with 18F-FDG PET and evaluation of diagnostic accuracy. J Nucl Med 47:904–911
**angsong Z, Changhong L, Weian C et al (2006) PET imaging of cerebral astrocytoma with 13N-ammonia. J Neurooncol 78:145–151. doi:10.1007/s11060-005-9069-x
Pöpperl G, Götz C, Rachinger W et al (2004) Value of O-(2-[18F]fluoroethyl)-l-tyrosine PET for the diagnosis of recurrent glioma. Eur J Nucl Med Mol Imaging 31:1464–1470. doi:10.1007/s00259-004-1590-1
Rachinger W, Goetz C, Pöpperl G et al (2005) Positron emission tomography with O-(2-[18F]fluoroethyl)-l-tyrosine versus magnetic resonance imaging in the diagnosis of recurrent gliomas. Neurosurgery 57:505–511. doi:10.1227/01.NEU.0000171642.49553.B0
Mehrkens JH, Pöpperl G, Rachinger W et al (2008) The positive predictive value of O-(2-[18F]fluoroethyl)-l-tyrosine (FET) PET in the diagnosis of a glioma recurrence after multimodal treatment. J Neurooncol 88:27–35. doi:10.1007/s11060-008-9526-4
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alexiou, G.A., Tsiouris, S., Kyritsis, A.P. et al. Glioma recurrence versus radiation necrosis: accuracy of current imaging modalities. J Neurooncol 95, 1–11 (2009). https://doi.org/10.1007/s11060-009-9897-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-009-9897-1