Abstract
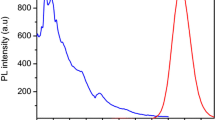
The sensitive and selective detection of mercury in aqueous solution is of paramount importance as the mercury concentration in drinking water above the threshold level set by world health organization can cause serious health issues to humans. We demonstrate a simple, facile, and cost-effective one pot synthesis route to synthesize MPA (3-mercaptopropionic acid) stabilized CdTe/CdS core-shell quantum dots and its application for detection of mercury using the fluorescence resonance energy transfer with a cationic dye, Rhodamine 6G. The quantum dots prepared via chemical reduction strategy using a combination of reducing agents, namely sodium borohydride and citric acid exhibit a high quantum efficiency (> 20% for solid state). Structural as well as luminescence studies of the prepared quantum dots were found to depend on the pH as well as the size of the quantum dots (hydrodynamic diameter ranging from 9 to 16 nm). Analysis of the fluorescence resonance energy transfer (FRET) between the prepared quantum dots and Rhodamine 6G elucidate that efficient energy transfer happens in the presence of a cetyl trimethyl ammonium bromide (CTAB) surfactant. Though the prepared CdTe/CdS quantum dots exhibit fluorescence quenching with an increase in mercury concentration and act as an “OFF-sensor,” the Rhodamine 6G-quantum dot pair employed here found to be a better approach as the inherent fluorescence of Rh6G is insensitive to mercury concentration. Our studies elucidate that the fluorescence ratio of Rh6G in a FRET pair follows a linear nature for the Stern-Volmer plot in the concentration range of Hg2+ ions (0.1 nM to 2 μM) and provide a LOD of 3.8 nM.

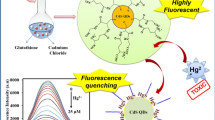
Schematic representation showing one-pot synthesis of CdTeCdS QDs for FRET based sensing of mercury ions in water.




Similar content being viewed by others
References
Aboulaich A, Billaud D, Abyan M, Balan L, Gaumet J-J, Medjadhi G, Ghanbaja J, Rl S (2012) One-pot noninjection route to CdS quantum dots via hydrothermal synthesis. ACS Appl Mater Interfaces 4:2561–2569
Ag D, Bongartz R, Dogan LE, Seleci M, Walter J-G, Demirkol DO, Stahl F, Ozcelik S, Timur S, Scheper T (2014) Biofunctional quantum dots as fluorescence probe for cell-specific targeting. Colloids Surf B: Biointerfaces 114:96–103
Akshya S, Hariharan P, Kumar VV, Anthony SP (2015) Surface functionalized fluorescent CdS QDs: selective fluorescence switching and quenching by Cu2+ and Hg2+ at wide pH range. Spectrochim Acta A Mol Biomol Spectrosc 135:335–341
Bao H, Gong Y, Li Z, Gao M (2004) Enhancement effect of illumination on the photoluminescence of water-soluble CdTe nanocrystals: toward highly fluorescent CdTe/CdS core-shell structure. Chem Mater 16:3853–3859
Basheer NS, Kumar BR, Kurian A, George SD (2013) Silver nanoparticle size-dependent measurement of quantum efficiency of Rhodamine 6G. Appl Phys B Lasers Opt 113:581–587
Brus L (1986) Zero-dimensional “excitons” in semiconductor clusters. IEEE J Quantum Electron 22:1909–1914
Chen G, Song F, **ong X, Peng X (2013) Fluorescent nanosensors based on fluorescence resonance energy transfer (FRET). Ind Eng Chem Res 52:11228–11245
Chen L, Zhao Q, Zhang X-Y, Tao G-H (2014) Determination of silver ion based on the redshift of emission wavelength of quantum dots functionalized with rhodanine. Chin Chem Lett 25:261–264
Chen Y, Chen Z, He Y, Lin H, Sheng P, Liu C, Luo S, Cai Q (2010) L-cysteine-capped CdTe QD-based sensor for simple and selective detection of trinitrotoluene. Nanotechnology 21:125502
Choi M-J, Pierson R, Chang Y, Guo H, Kang I-K (2013) Enhanced intracellular uptake of CdTe quantum dots by conjugation of oligopeptides. J Nanomater 2013:14
Chu VH, Nghiem THL, Le TH, Vu DL, Tran HN, Vu TKL (2012) Synthesis and optical properties of water soluble CdSe/CdS quantum dots for biological applications. Adv Nat Sci Nanosci Nanotechnol 3:025017
Clapp AR, Medintz IL, Mauro JM, Fisher BR, Bawendi MG, Mattoussi H (2004) Fluorescence resonance energy transfer between quantum dot donors and dye-labeled protein acceptors. J Am Chem Soc 126:301–310
de Mello DC (2011) Synthesis and properties of colloidal heteronanocrystals. Chem Soc Rev 40:1512–1546
De Sousa ME, Fernández van Raap MB, Rivas PC, Mendoza Zélis P, Girardin P, Pasquevich GA, Alessandrini JL, Muraca D, Sánchez FH (2013) Stability and relaxation mechanisms of citric acid coated magnetite nanoparticles for magnetic hyperthermia. J Phys Chem C 117:5436–5445
de Souza GC, Ribeiro DS, Rodrigues SSM, Paim APS, Lavorante AF, da Silva VL, Santos JL, Araújo AN, Montenegro MCB (2016) Clean photoinduced generation of free reactive oxygen species by silica films embedded with CdTe–MTA quantum dots. RSC Adv 6:8563–8571
Ding X, Qu L, Yang R, Zhou Y, Li J (2015) A highly selective and simple fluorescent sensor for mercury (II) ion detection based on cysteamine-capped CdTe quantum dots synthesized by the reflux method. Luminescence 30:465–471
Duan J, Song L, Zhan J (2009) One-pot synthesis of highly luminescent CdTe quantum dots by microwave irradiation reduction and their Hg2+-sensitive properties. J Nano Res 2:61–68
Emin S, Loukanov A, Wakasa M, Nakabayashi S, Kaneko Y (2010) Photostability of water-dispersible CdTe quantum dots: cap** ligands and oxygen. Chem Lett 39:654–656
Frasco MF, Chaniotakis N (2009) Semiconductor quantum dots in chemical sensors and biosensors. Sensors 9:7266–7286
Gaponik N, Talapin DV, Rogach AL, Hoppe K, Shevchenko EV, Kornowski A, Eychmüller A, Weller H (2002) Thiol-cap** of CdTe nanocrystals: an alternative to organometallic synthetic routes. J Phys Chem B 106:7177–7185
Geyer S, Porter VJ, Halpert JE, Mentzel TS, Kastner MA, Bawendi MG (2010) Charge transport in mixed CdSe and CdTe colloidal nanocrystal films. Phys Rev B 82:155201
Gong T, Liu J, Liu X, Liu J, **ang J, Wu Y (2016) A sensitive and selective sensing platform based on CdTe QDs in the presence of l-cysteine for detection of silver, mercury and copper ions in water and various drinks. Food Chem 213:306–312
Huang F, Huang B (2015) Aqueous synthesis of water-soluble citrate-modified cadmium selenide/cadmium sulfide/zinc sulfide quantum dots. Spectrosc Lett 48:422–426
**g L, Kershaw SV, Li Y, Huang X, Li Y, Rogach AL, Gao M (2016) Aqueous based semiconductor nanocrystals. Chem Rev 116:10623–10730
John J, Thomas L, George NA, Kurian A, George SD (2015) Tailoring of optical properties of fluorescein using green synthesized gold nanoparticles. Phys Chem Chem Phys 17:15813–15821
Kumar MMD, Devadason S (2013) Structural and optical properties of CdTe/CdSe heterostructure multilayer thin films prepared by physical vapor deposition technique. S Appl Nanosci 3:453–459
Kumar P, Kim K-H, Bansal V, Lazarides T, Kumar N (2017) Progress in the sensing techniques for heavy metal ions using nanomaterials. J Ind Eng Chem 54:30–43
Kurian A, George SD, Nampoori V, Vallabhan C (2005) Study on the determination of molecular distance in organic dye mixtures using dual beam thermal lens technique. Spectrochim Acta A Mol Biomol Spectrosc 61:2799–2802
Li H, Shih WY, Shih W-H (2007) Synthesis and characterization of aqueous carboxyl-capped CdS quantum dots for bioapplications. Ind Eng Chem Res 46:2013–2019
Li J, Mei F, Li W-Y, He X-W, Zhang Y-K (2008) Study on the fluorescence resonance energy transfer between CdTe QDs and butyl-rhodamine B in the presence of CTMAB and its application on the detection of Hg(II). Spectrochim Acta A Mol Biomol Spectrosc 70:811–817
Martin A, Narayanaswamy R (1997) Studies on quenching of fluorescence of reagents in aqueous solution leading to an optical chloride-ion sensor. Sensors Actuators B Chem 39:330–333
Mathew S, Saran AD, Singh Bhardwaj B, Ani Joseph S, Radhakrishnan P, Nampoori V, Vallabhan C, Bellare JR (2012) Size dependent optical properties of the CdSe-CdS core-shell quantum dots in the strong confinement regime. J Appl Phys 111:074312
Medintz IL, Clapp AR, Mattoussi H, Goldman ER, Fisher B, Mauro JM (2003) Self-assembled nanoscale biosensors based on quantum dot FRET donors. Nat Mater 2:630–638
Murray C, Norris DJ, Bawendi MG (1993) Synthesis and characterization of nearly monodisperse CdE (E= sulfur, selenium, tellurium) semiconductor nanocrystallites. J Am Chem Soc 115:8706–8715
Paim APS, Rodrigues SSM, Ribeiro DS, de Souza GC, Santos JL, Araújo AN, Amorim CG, Teixeira-Neto É, da Silva VL, Montenegro MC (2017) Fluorescence probe for mercury (ii) based on the aqueous synthesis of CdTe quantum dots stabilized with 2-mercaptoethanesulfonate. New J Chem 41:3265–3272
Piella J, Bastús NG, Puntes V (2016) Size-controlled synthesis of sub-10-nanometer citrate-stabilized gold nanoparticles and related optical properties. Chem Mater 28:1066–1075
Rajh T, Micic OI, Nozik AJ (1993) Synthesis and characterization of surface-modified colloidal cadmium telluride quantum dots. J Phys Chem 97:11999–12003
Resch-Genger U, Grabolle M, Cavaliere-Jaricot S, Nitschke R, Nann T (2008) Quantum dots versus organic dyes as fluorescent labels. Nat Methods 5:763–775
Rodzik-Czałka Ł, Lewandowska-Łańcucka J, Gatta V, Venditti I, Fratoddi I, Szuwarzyński M, Romek M, Nowakowska M (2017) Nucleobases functionalized quantum dots and gold nanoparticles bioconjugates as a fluorescence resonance energy transfer (FRET) system—synthesis, characterization and potential applications. J Colloid Interface Sci
Saikia D, Dutta P, Sarma NS, Adhikary NC (2016) CdTe/ZnS core/shell quantum dot-based ultrasensitive PET sensor for selective detection of Hg(II) in aqueous media. Sensors Actuators B Chem 230:149–156
Shamirian A, Ghai A, Snee PT (2015) QD-based FRET probes at a glance. Sensors 15:13028–13051
Singh H, Saleem SM, Singh R, Birdi K (1980) Micelle formation of ionic surfactants in polar nonaqueous solvents. J Phys Chem 84:2191–2194
Smith AM, Mohs AM, Nie S (2009) Tuning the optical and electronic properties of colloidal nanocrystals by lattice strain. Nat Nanotechnol 4:56–63
Tang G, Du L, Su X (2013) Detection of melamine based on the fluorescence resonance energy transfer between CdTe QDs and Rhodamine B. Food Chem 141:4060–4065
Tang G, Wang J, Li Y, Su X (2015) Determination of arsenic (III) based on the fluorescence resonance energy transfer between CdTe QDs and Rhodamine 6G. RSC Adv 5:17519–17525
Tang L, Mo S, Liu SG, Ling Y, Zhang XF, Li NB, Luo HQ (2018) A sensitive “turn-on” fluorescent sensor for melamine based on FRET effect between polydopamine-glutathione nanoparticles and Ag nanoparticles. J Agric Food Chem 66:2174–2179
Ullah N, Mansha M, Khan I, Qurashi A (2018) Nanomaterial-based optical chemical sensors for the detection of heavy metals in water: recent advances and challenges. TrAC Trends Anal Chem 100:155–166
Ulusoy M, Walter J-G, Lavrentieva A, Kretschmer I, Sandiford L, Le Marois A, Bongartz R, Aliuos P, Suhling K, Stahl F (2015) One-pot aqueous synthesis of highly strained CdTe/CdS/ZnS nanocrystals and their interactions with cells. RSC Adv 5:7485–7494
Vale BR, Silva FO, Carvalho MS, Raphael E, Ferrari JL, Schiavon MA (2016) Water-soluble CdTe/CdS Core/Shell semiconductor nanocrystals: how their optical properties depend on the synthesis methods. Crystals 6:133
Vázquez-González M, Carrillo-Carrion C (2014) Analytical strategies based on quantum dots for heavy metal ions detection. J Biomed Opt 19:101503
Wang Y-Y, **ang X, Yan R, Liu Y, Jiang F-L (2018) Förster resonance energy transfer from quantum dots to rhodamine B as mediated by a cationic surfactant: a thermodynamic perspective. J Phys Chem C 122:1148–1157
Yao J, Gou X (2016) An investigation of preparation, properties, characterization and the mechanism of zinc blende CdTe/CdS core/shell quantum dots for sensitive and selective detection of trace mercury. J Mater Chem C 4:9856–9863
Yu WW, Qu L, Guo W, Peng X (2003) Experimental determination of the extinction coefficient of CdTe, CdSe, and CdS nanocrystals. Chem Mater 15:2854–2860
Zhang Y-Y, Kim J-Y, Kim Y, Jang D-J (2012) Controlled optical properties of water-soluble CdTe/CdS/ZnS quantum dots. J Nanopart Res 14:1117
Zhou D, Lin M, Chen Z, Sun H, Zhang H, Sun H, Yang B (2011) Simple synthesis of highly luminescent water-soluble CdTe quantum dots with controllable surface functionality. Chem Mater 23:4857–4862
Zhu J, Zhao Z-J, Li J-J, Zhao J-W (2017) CdTe quantum dot-based fluorescent probes for selective detection of Hg(II): the effect of particle size. Spectrochim Acta, Part A 177:140–146
Zrazhevskiy P, Gao X (2013) Quantum dot imaging platform for single-cell molecular profiling. Nat Commun 4:1619
Acknowledgements
We gratefully acknowledge the (1) DST-FIST programme via approval letter SR/FST/PSI-174/2012 for providing Powder X-ray diffractometer; (2) SERB, DST Govt. of India through the project no. SB/S2/CMP-017/2014; (3) SAIF, IIT Bombay, for providing HR-TEM facility; (4) Dr. Sudarshan Kini, sincerely thanks Manipal University for providing the postdoctoral fellowship; and (5) SDG acknowledges Manipal University for Dr. T M A Pai Endowment Chair in Applied Nanosciences.
Funding
This study received financial support from Joint Manipal University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 1886 kb)
Rights and permissions
About this article
Cite this article
Kini, S., Ganiga, V., Kulkarni, S.D. et al. Sensitive detection of mercury using the fluorescence resonance energy transfer between CdTe/CdS quantum dots and Rhodamine 6G. J Nanopart Res 20, 232 (2018). https://doi.org/10.1007/s11051-018-4320-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-018-4320-5