Abstract
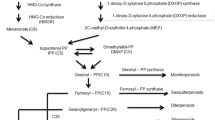
Isoprenoids or terpenoids are synthesized by two important units’ including dimethylallyl diphosphate and isopentenyl diphosphate (IPP). Plants use two different methods for formation of IPP, which is a cytosolic and a plastidial method. The 3-hydroxy-3-methylglutaryl-CoA reductase (HMGR, EC 1.1.1.34) catalyzes the conversion of HMG-CoA to mevalonate, which is the first stage in the cytosolic pathway for biosynthesis of isoprenoid in plants. In this study, a total of fifty HMGR protein sequences from Gramineae and three animal samples including human, mouse and fruit fly were aligned and analyzed by computational tools to predict the protein properties, such as molecular mass, pI, signal peptide, transmembrane and conserved domains, secondary and spatial structures. Sequence comparison analysis revealed that there is high identity between plants and animals. Three catalytic regions including L domain, N domain and S domain were detected by structural modeling of HMGR. The tertiary structure model of Oryza sativa HMGR (Accession Number: NP_001063541) was further checked by PROCHECK algorithm, and showed that 90.3 % of the amino acid residues were located in the most favored regions in Ramachandran plot, indicating that the simulated three-dimensional structure was reliable. Phylogenetic analysis indicated that there is a relationship among species of Gramineae and other organisms. According to these results, HMGRs should be derived from a common ancestor.









Similar content being viewed by others
Abbreviations
- CAS:
-
Cycloartenol synthase
- DMAPP:
-
Dimethylallyl diphosphate
- DXP:
-
1-Deoxy-d-xylulose 5-phosphate
- HMGR:
-
3-Hydroxy-3-methylglutaryl coenzyme A reductase
- IPP:
-
Isopentenyl diphosphate
- LAS:
-
Lanosterol synthase
- MEP:
-
2C-methyl-d-erythritol 4-phosphate
- MVA:
-
Mevalonic acid
- OSC:
-
Oxidosqualene cyclase
- UDP:
-
Uridine diphosphate
- UGT:
-
(UDP)-dependent glycosyltransferases
References
Abe I, Rohmer M, Prestwich GD (1993) Enzymatic cyclization of squalene and oxido squalene to sterols and triterpenes. Chem Rev 93:2189–2206
Bailey TL, Williams N, Misleh C, Li WW (2006) MEME: discovering and analyzing DNA and protein sequence motifs. Nucleic Acids Res 34:369–373
Bick JA, Lange BM (2003) Metabolic cross talk between cytosolic and plastidial pathways of isoprenoid biosynthesis: unidirectional transport of intermediates across the chloroplast envelope membrane. Arch Biochem Biophys 415:146–154
Caelles C, Ferrer A, Balcells L, Hegardt FG, Boronat A (1989) Isolation and structural characterization of a cDNA encoding Arabidopsis thaliana 3-hydroxy-3-methylglutaryl coenzyme A reductase. Plant Mol Biol 13:627–638
Chappell J, Wolf F, Proulx J, Cuellar R, Saunders C (1995) Is the reaction catalyzed by 3-hydroxy-3-methylglutaryl coenzyme A reductase a rate-limiting step for isoprenoid biosynthesis in plants? Plant Physiol 109:1337–1343
Chen XY, Xu ZH (1996) Recent progress in biotechnology and genetic engineering of medicinal plants in China. Med Chem Res 6:215–224
Cieza A, Maier P, Poppel E (2003) The effect of Ginkgo Biloba on healthy elderly subjects. Fortschritte Der Medzin 1:10–15
Croteau R, Kutchan TM, Lewis NG (2000) Natural products (secondary metabolites). In: Buchanan B, Gruissem W, Jones R (eds) Biochemistry and molecular biology of plants. Am Soc Plant Biologists, Rockville, MD, USA, Chapter 24, pp 1255–1318
Eriksson ME, Israelsson M, Olsson O, Mortiz T (2000) Increased gibberellins biosynthesis in transgenic trees promotes growth, biomass production and xylem fiber length. Nat Biotechnol 18:784–788
Fits LVD, Memelink J (2000) ORCA3, a jasmonate-responsive transcriptional regulator of plant primary and secondary metabolism. Science 289:295–297
Geourjon C, Dele′age G (1995) SOPMA: significant improvement in protein secondary structure prediction by consensus prediction from multiple alignments. CABIOS 11:681–684
Harker M, Holmberg N, Clayton JC, Gibbard CL, Wallace AD, Rawlins S, Hellyer SA, Lanot A, Safford R (2003) Enhancement of seed phytosterol levels by expression of an N-terminal truncated Hevea brasiliensis (rubber tree) 3-hydroxy- 3-methylglutaryl-CoA reductase. Plant Biotechnol J 1:113–121
Hartmann T (1996) Diversity and variability of plant secondary metabolism: a mechanistic view. Entomol Gen Appl 80:177–188
Hey SJ, Powers SJ, Beale MH, Hawkins ND, Ward JL, Halford NG (2006) Enhanced seed phytosterol accumulation through expression of a modified HMG-CoA reductase. Plant Biotechnol J 4:219–229
Istvan ES, Deisenhofer J (2000) The structure of the catalytic portion of human HMG-CoA reductase. Biochem Biophys Acta 1529:9–18
Jomaa H, Wiesner J, Sanderbrand S, Altincicek B, Weidemeyer K, Hintz M, Turbachova I, Eberl M, Zeidler J, Lichtenthaler HK, Soldati D, Beck E (1999) Inhibitors of the nonmevalonate pathway of isoprenoid biosynthesis as antimaterial drugs. Science 285:1573–1576
Kahn RA, Durst F (2000) Function and evolution of plant cytochrome P450. Recent Adv Phytochem 34:151–189
Kushiro T, Ebizuka Y (2010) Triterpene. Compr Nat Prod II: Chem Biol 1:673–708
Lambert C, Leonard N, De Bolle X, Depiereux E (2002) ESyPred3D: prediction of proteins 3D structures. Bioinforma 18:1250–1256
Lange BM, Croteau R (1999) Genetic engineering of essential oil production in mint. Curr Opin Plant Biol 2:139–144
Lange BM, Ketchum REB, Croteau R (2001) Isoprenoid biosynthesis: metabolite profiling of Peppermint oil gland secretory cells and application to herbicide target analysis. Plant Physiol 127:305–314
Laskowski RA, MacArthur MW, Moss DS, Thornton JM (1993) PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Crystallogr 26:283–291
Mann V, Harker M, Pecker I, Hirschberg J (2000) Metabolic engineering of astaxanthin production in tobacco flowers. Nat Biotechnol 18:888–892
Manzano D, Fernández-Busquets X, Schaller H, González V, Boronat A, Arró M, Ferrer A (2004) The metabolic imbalance underlying lesion formation in Arabidopsis thaliana overexpressing farnesyl diphosphate synthase (isoform 1S) leads to oxidative stress and is triggered by the developmental decline of endogenous HMGR activity. Planta 219:982–989
Martin VJJ, Pitera DJ, Withers ST, Newman JD, Keasling JD (2003) Engineering a mevalonate pathway in Escherichia coli for production of terpenoids. Nat Biotech 21:796–802
Matthews PD, Wurtzel ET (2000) Metabolic engineering of carotenoid accumulation in Escherichia coli by modulation of the isoprenoid precursor pool with expression of deoxyxylulose phosphate synthase. Appl Microbiol Biotechnol 53:396–400
Muñoz-Bertomeu J, Sales E, Ros R, Arrillaga I, Segura J (2007) Up-regulation of an N-terminal truncated 3-hydroxy-3-methylglutaryl CoA reductase enhances production of essential oils and sterols in transgenic Lavandula latifolia. Plant Biotechnol J 5:746–758
Ohyama K, Suzuki M, Kikuchi J, Saito K, Muranaka T (2009) Dual biosynthetic pathways to phytosterol via cycloartenol and lanosterol in Arabidopsis. Proc Natl Acad Sci USA 106:725–730
Romer S, Bramley PM, Fraser PD, Kiano JW, Shipton CA, Misawa N, Schuch W (2000) Elevation of the provitamin A content of transgenic tomato plants. Nat Biotechnol 18:666–669
Ruiz-Albert J, Cerda-Olmedo E, Corrochano LM (2001) Genes for mevalonate biosynthesis in phycomyces. Mol Genet Genomics 266:768–777
Schaller H, Grausem B, Benveniste P, Chye ML, Tan CT, Song YH, Chua NH (1995) Expression of the Hevea brasiliensis (HBK) Müll Arg 3-hydroxy-3-methylglutaryl coenzyme A reductase 1 in tobacco results in sterol overproduction. Plant Physiol 109:761–770
Schmidt-Dannert C, Umeno D, Arnold FH (2000) Molecular breeding of carotenoid biosynthetic pathways. Nat Biotechnol 18:750–753
Soriente A, De Rosa M, Scettri A, Sodano G, Terenico MC, Payá M, Alcaraz MJ (1999) Manolide. Curr Med Chem 6:415–431
Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT (1971) Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc 93:2325–2327
Xu R, Fazio GC, Matsuda SPT (2004) On the origins of triterpenoid skeletal diversity. Phytochemistry 65:261–291
Ye XD, Babili SA, Klöti A, Zhang J, Lucca P, Beyer P, Potrykus I (2000) Engineering provitamin A (β-carotene) biosynthetic pathway into (caroteneoid free) rice endosperm. Science 287:303–305
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s11033-015-3872-z.
Rights and permissions
About this article
Cite this article
Darabi, M., Masoudi-Nejad, A. & Nemat-Zadeh, G. Bioinformatics study of the 3-hydroxy-3-methylglotaryl-coenzyme A reductase (HMGR) gene in Gramineae. Mol Biol Rep 39, 8925–8935 (2012). https://doi.org/10.1007/s11033-012-1761-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-012-1761-2