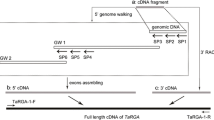
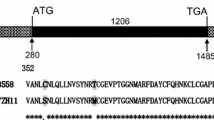
Abstract
Bacterial leaf streak of rice (BLS) caused by Xanthomonas oryzae pv. oryzicola (Xoc) is a widely-spread disease in the main rice-producing areas of the world. Investigating the genes that play roles in rice–Xoc interactions helps us to understand the defense signaling pathway in rice. Here we report a differentially expressed protein gene (DEPG1), which regulates susceptibility to BLS. DEPG1 is a nucleotide-binding site (NBS)-leucine rich repeat (LRR) gene, and the deduced protein sequence of DEPG1 has approximately 64% identity with that of the disease resistance gene Pi37. Phylogenetic analysis of DEPG1 and the 18 characterized NBS-LRR genes revealed that DEPG1 is more closely related to Pi37. DEPG1 protein is located to the cytoplasm, which was confirmed by transient expression of DEPG1-GFP (green fluorescent protein) fusion construct in onion epidermal cells. Semi-quantitative PCR assays showed that DEPG1 is widely expressed in rice, and is preferentially expressed in internodes, leaf blades, leaf sheaths and flag leaves. Observation of cross sections of leaves from the transgenic plants with a DEPG1-promoter::glucuronidase (GUS) fusion gene revealed that DEPG1 is also highly expressed in mesophyll tissues where Xoc mainly colonizes. Additionally, Xoc negatively regulates expression of DEPG1 at the early stage of the pathogen infection, and so do the three defense-signal compounds including salicylic acid (SA), methyl jasmonate (MeJA) and 1-aminocyclopropane-1-carboxylic-acid (ACC). Transgenic rice plants overexpressing DEPG1 exhibit enhanced susceptibility to Xoc compared to the wild-type controls. Moreover, enhanced susceptibility to Xoc may be mediated by inhibition of the expression of some SA biosynthesis-related genes and pathogenesis-related genes that may contribute to the disease resistance. Taken together, DEPG1 plays roles in the interactions between rice and BLS pathogen Xoc.











Similar content being viewed by others
Abbreviations
- ACC:
-
1-Aminocyclopropane-1-carboxylic acid
- CTAB:
-
Cetyltrimethyl ammonium bromide
- ET:
-
Ethylene
- GFP:
-
Green fluorescent protein
- JA:
-
Jasmonic acid
- MeJA:
-
Methyl jasmonate
- MALDI-TOF-MS:
-
Matrix assisted laser desorption ionization-time of flight-mass spectrometry
- NBS-LRR:
-
Nucleotide binding site-leucine rich repeat
- ORF:
-
Open reading frame
- QTL:
-
Quantitative trait locus
- RT-PCR:
-
Reverse transcription PCR
- SA:
-
Salicylic acid
- Xoo:
-
Xanthomonas oryzae pv. oryzae
- Xoc:
-
Xanthomonas oryzae pv. oryzicola
References
Dai LY, Liu XL, **ao YH, Wang GL (2007) Recent advances in cloning and characterization of disease resistance genes in rice. J Integr Plant Biol 49(1):112–119. doi:10.1111/j.1672-9072.2007.00413.x
Tang D, Wu W, Li W, Lu H, Worland AJ (2000) Map** of QTLs conferring resistance to bacterial leaf streak in rice. Theor Appl Genet 101(1):286–291. doi:10.1007/s001220051481
Fu J, Liu H, Li Y, Yu H, Li X, **ao J, Wang S (2010) Manipulating broad-spectrum disease resistance by suppressing pathogen-induced auxin accumulation in rice. Plant Physiol. doi:10.1104/pp.110.163774
Tao Z, Liu H, Qiu D, Zhou Y, Li X, Xu C, Wang S (2009) A pair of allelic WRKY genes play opposite roles in rice–bacteria interactions. Plant Physiol 151(2):936–948. doi:10.1104/pp.109.145623
Shen X, Yuan B, Liu H, Li X, Xu C, Wang S (2010) Opposite functions of a rice mitogen-activated protein kinase during the process of resistance against Xanthomonas oryzae. Plant J 64(1):86–99. doi:10.1111/j.1365-313X.2010.04306.x
Zhao B, Lin X, Poland J, Trick H, Leach J, Hulbert S (2005) A maize resistance gene functions against bacterial streak disease in rice. Proc Natl Acad Sci USA 102(43):15383–15388. doi:10.1073/pnas.0503023102
Bryan GT, Wu KS, Farrall L, Jia Y, Hershey HP, McAdams SA, Faulk KN, Donaldson GK, Tarchini R, Valent B (2000) tA single amino acid difference distinguishes resistant and susceptible alleles of the rice blast resistance gene Pi-ta. Plant Cell 12(11):2033–2046
Wang ZX, Yano M, Yamanouchi U, Iwamoto M, Monna L, Hayasaka H, Katayose Y, Sasaki T (1999) The Pib gene for rice blast resistance belongs to the nucleotide binding and leucine-rich repeat class of plant disease resistance genes. Plant J 19(1):55–64. doi:10.1046/j.1365-313X.1999.00498.x
Zhou B, Qu SH, Liu GF, Dolan M, Sakai H, Lu GD, Bellizzi M, Wang GL (2006) The eight amino-acid differences within three leucine-rich repeats between Pi2 and Piz-t resistance proteins determine the resistance specificity to Magnaporthe grisea. Mol Plant Microbe Interact 19(11):1216–1228. doi:10.1094/Mpmi-19-1216
Qu SH, Liu GF, Zhou B, Bellizzi M, Zeng LR, Dai LY, Han B, Wang GL (2006) The broad-spectrum blast resistance gene Pi9 encodes a nucleotide-binding site-leucine-rich repeat protein and is a member of a multigene family in rice. Genetics 172(3):1901–1914. doi:10.1534/genetics.105.044891
Lin F, Chen S, Que ZQ, Wang L, Liu XQ, Pan QH (2007) The blast resistance gene Pi37 encodes a nucleotide binding site-leucine-rich repeat protein and is a member of a resistance gene cluster on rice chromosome 1. Genetics 177(3):1871–1880. doi:10.1534/genetics.107.080648
Liu X, Lin F, Wang L, Pan Q (2007) The in silico map-based cloning of Pi36, a rice coiled-coil nucleotide-binding site leucine-rich repeat gene that confers race-specific resistance to the blast fungus. Genetics 176(4):2541–2549. doi:10.1534/genetics.107.075465
Ashikawa I, Hayashi N, Yamane H, Kanamori H, Wu J, Matsumoto T, Ono K, Yano M (2008) Two adjacent nucleotide-binding site-leucine-rich repeat class genes are required to confer Pikm-specific rice blast resistance. Genetics 180(4):2267–2276. doi:genetics.108.095034
Hayashi K, Yasuda N, Fujita Y, Koizumi S, Yoshida H (2010) Identification of the blast resistance gene Pit in rice cultivars using functional markers. Theor Appl Genet 121(7):1357–1367. doi:10.1007/s00122-010-1393-7
Shang J, Tao Y, Chen X, Zou Y, Lei C, Wang J, Li X, Zhao X, Zhang M, Lu Z, Xu J, Cheng Z, Wan J, Zhu L (2009) Identification of a new rice blast resistance gene, Pid3, by genome wide comparison of paired nucleotide-binding site—leucine-rich repeat genes and their pseudogene alleles between the two sequenced rice genomes. Genetics 182(4):1303–1311. doi:10.1534/genetics.109.102871
Hayashi N, Inoue H, Kato T, Funao T, Shirota M, Shimizu T, Kanamori H, Yamane H, Hayano-Saito Y, Matsumoto T, Yano M, Takatsuji H (2010) Durable panicle blast-resistance gene Pb1 encodes an atypical CC-NBS-LRR protein and was generated by acquiring a promoter through local genome duplication. Plant J 64(3):498–510. doi:10.1111/j.1365-313X.2010.04348.x
Yoshimura S, Yamanouchi U, Katayose Y, Toki S, Wang ZX, Kono I, Kurata N, Yano M, Iwata N, Sasaki T (1998) Expression of Xa1, a bacterial blight-resistance gene in rice, is induced by bacterial inoculation. Proc Natl Acad Sci USA 95(4):1663–1668
Faigon-Soverna A, Harmon FG, Storani L, Karayekov E, Staneloni RJ, Gassmann W, Mas P, Casal JJ, Kay SA, Yanovsky MJ (2006) A constitutive shade-avoidance mutant implicates TIR-NBS-LRR proteins in Arabidopsis photomorphogenic development. Plant Cell 18(11):2919–2928. doi:10.1105/tpc.105.038810
Hewezi T, Mouzeyar S, Thion L, Rickauer M, Alibert G, Nicolas P, Kallerhoff J (2006) Antisense expression of a NBS-LRR sequence in sunflower (Helianthus annuus L.) and tobacco (Nicotiana tabacum L.): evidence for a dual role in plant development and fungal resistance. Transgenic Res 15(2):165–180. doi:10.1007/s11248-005-3518-3
Zhai C, Lin F, Dong Z, He X, Yuan B, Zeng X, Wang L, Pan Q (2011) The isolation and characterization of Pik, a rice blast resistance gene which emerged after rice domestication. New Phytol 189(1):321–334. doi:10.1111/j.1469-8137.2010.03462.x
Zou LF, Wang XP, **ang Y, Zhang B, Li YR, **ao YL, Wang JS, Walmsley AR, Chen GY (2006) Elucidation of the hrp clusters of Xanthomonas oryzae pv. oryzicola that control the hypersensitive response in nonhost tobacco and pathogenicity in susceptible host rice. Appl Environ Microbiol 72(9):6212–6224. doi:10.1128/AEM.00511-06
Zhou YL, Xu MR, Zhao MF, **e XW, Zhu LH, Fu BY, Li ZK (2010) Genome-wide gene responses in a transgenic rice line carrying the maize resistance gene Rxo1 to the rice bacterial streak pathogen, Xanthomonas oryzae pv. oryzicola. BMC Genomics 11:78. doi:10.1186/1471-2164-11-78
Xu RR, Song FM, Zheng Z (2006) OsBISAMT1, a gene encoding S-adenosyl-l-methionine: salicylic acid carboxyl methyltransferase, is differentially expressed in rice defense responses. Mol Biol Rep 33(3):223–231. doi:10.1007/s11033-005-4823-x
Hofgen R, Willmitzer L (1988) Storage of competent cells for Agrobacterium transformation. Nucleic Acids Res 16(20):9877. doi:10.1093/nar/16.20.9877
Curtis MD, Grossniklaus U (2003) A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol 133(2):462–469. doi:10.1104/pp.103.027979
Nishimura A, Aichi I, Matsuoka M (2006) A protocol for Agrobacterium-mediated transformation in rice. Nat Protoc 1(6):2796–2802. doi:10.1038/nprot.2006.469
Yuan M, Chu Z, Li X, Xu C, Wang S (2010) The bacterial pathogen Xanthomonas oryzae overcomes rice defenses by regulating host copper redistribution. Plant Cell 22(9):3164–3176. doi:10.1105/tpc.110.078022
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) Software Version 4.0. Mol Biol Evol 24(8):1596–1599. doi:10.1093/molbev/msm092
Traut TW (1994) The functions and consensus motifs of nine types of peptide segments that form different types of nucleotide-binding sites. Eur J Biochem 222(1):9–19. doi:10.1111/j.1432-1033.1994.tb18835.x
Sun Q, Collins NC, Ayliffe M, Smith SM, Drake J, Pryor T, Hulbert SH (2001) Recombination between paralogues at the rp1 rust resistance locus in maize. Genetics 158(1):423–438
Halterman D, Zhou F, Wei F, Wise RP, Schulze-Lefert P (2001) The MLA6 coiled-coil, NBS-LRR protein confers AvrMla6-dependent resistance specificity to Blumeria graminis f. sp. hordei in barley and wheat. Plant J 25(3):335–348. doi:10.1046/j.1365-313x.2001.00982.x
Zhou F, Kurth J, Wei F, Elliott C, Vale G, Yahiaoui N, Keller B, Somerville S, Wise R, Schulze-Lefert P (2001) Cell-autonomous expression of barley Mla1 confers race-specific resistance to the powdery mildew fungus via a Rar1-independent signaling pathway. Plant Cell 13(2):337–350. doi:10.1105/tpc.13.2.337
Lawrence GJ, Finnegan EJ, Ayliffe MA, Ellis JG (1995) The L6 gene for flax rust resistance is related to the Arabidopsis bacterial resistance gene RPS2 and the tobacco viral resistance gene N. Plant Cell 7(8):1195–1206. doi:10.1105/tpc.7.8.1195
Parker JE, Coleman MJ, Szabo V, Frost LN, Schmidt R, van der Biezen EA, Moores T, Dean C, Daniels MJ, Jones JD (1997) The Arabidopsis downy mildew resistance gene RPP5 shares similarity to the toll and interleukin-1 receptors with N and L6. Plant Cell 9(6):879–894. doi:10.1105/tpc.9.6.879
Whitham S, Dinesh-Kumar SP, Choi D, Hehl R, Corr C, Baker B (1994) The product of the tobacco mosaic virus resistance gene N: similarity to toll and the interleukin-1 receptor. Cell 78(6):1101–1115. doi:10.1016/0092-8674(94)90283-6
Mitsuhara I, Iwai T, Seo S, Yanagawa Y, Kawahigasi H, Hirose S, Ohkawa Y, Ohashi Y (2008) Characteristic expression of twelve rice PR1 family genes in response to pathogen infection, wounding, and defense-related signal compounds (121/180). Mol Genet Genomics 279(4):415–427. doi:10.1007/s00438-008-0322-9
Nakashita H, Yoshioka K, Takayama M, Kuga R, Midoh N, Usami R, Horikoshi K, Yoneyama K, Yamaguchi I (2001) Characterization of PBZ1, a probenazole-inducible gene, in suspension-cultured rice cells. Biosci Biotechnol Biochem 65(1):205–208. doi:10.1271/bbb.65.205
Qiu D, **ao J, Ding X, **ong M, Cai M, Cao Y, Li X, Xu C, Wang S (2007) OsWRKY13 mediates rice disease resistance by regulating defense-related genes in salicylate- and jasmonate-dependent signaling. Mol Plant Microbe Interact 20(5):492–499. doi:10.1094/MPMI-20-5-0492
Zhao CJ, Wang AR, Shi YJ, Wang LQ, Liu WD, Wang ZH, Lu GD (2008) Identification of defense-related genes in rice responding to challenge by Rhizoctonia solani. Theor Appl Genet 116(4):501–516. doi:10.1007/s00122-007-0686-y
Datta K, Velazhahan R, Oliva N, Ona I, Mew T, Khush GS, Muthukrishnan S, Datta SK (1999) Over-expression of the cloned rice thaumatin-like protein (PR-5) gene in transgenic rice plants enhances environmental friendly resistance to Rhizoctonia solani causing sheath blight disease. Theor Appl Genet 98(6):1138–1145. doi:10.1007/s001220051178
Hashimoto M, Kisseleva L, Sawa S, Furukawa T, Komatsu S, Koshiba T (2004) A novel rice PR10 protein, RSOsPR10, specifically induced in roots by biotic and abiotic stresses, possibly via the jasmonic acid signaling pathway. Plant Cell Physiol 45(5):550–559. doi:10.1093/pcp/pch063
Bari R, Jones JD (2009) Role of plant hormones in plant defence responses. Plant Mol Biol 69(4):473–488. doi:10.1007/s11103-008-9435-0
Vlot AC, Dempsey DA, Klessig DF (2009) Salicylic Acid, a multifaceted hormone to combat disease. Annu Rev Phytopathol 47:177–206. doi:10.1146/annurev.phyto.050908.135202
Song FM, Goodman RM (2001) Molecular biology of disease resistance in rice. Physiol Mol Plant Pathol 59(1):1–11. doi:10.1006/pmpp.2001.0353
DeYoung BJ, Innes RW (2006) Plant NBS-LRR proteins in pathogen sensing and host defense. Nat Immunol 7(12):1243–1249. doi:10.1038/ni1410
McHale L, Tan X, Koehl P, Michelmore RW (2006) Plant NBS-LRR proteins: adaptable guards. Genome Biol 7(4):212. doi:10.1186/gb-2006-7-4-212
Zhou T, Wang Y, Chen JQ, Araki H, **g Z, Jiang K, Shen J, Tian D (2004) Genome-wide identification of NBS genes in japonica rice reveals significant expansion of divergent non-TIR NBS-LRR genes. Mol Genet Genomics 271(4):402–415. doi:10.1007/s00438-004-0990-z
Nino-Liu DO, Ronald PC, Bogdanove AJ (2006) Xanthomonas oryzae pathovars: model pathogens of a model crop. Mol Plant Pathol 7(5):303–324. doi:10.1111/j.1364-3703.2006.00344.x
Acknowledgments
The authors acknowledge Mingfu Zhao, Fujian Academy of Agricultural Sciences for providing us with the bacterial pathogen Xanthomonas oryzae pv. oryzicola RS105 strain. We also would like to thank National 863 Project of China (Grant No. 2007AA10Z132 and 2009ZX08009-045B) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Lijia Guo and Min Li contributed equally to this work.
Rights and permissions
About this article
Cite this article
Guo, L., Li, M., Wang, W. et al. Over-expression in the nucleotide-binding site-leucine rich repeat gene DEPG1 increases susceptibility to bacterial leaf streak disease in transgenic rice plants. Mol Biol Rep 39, 3491–3504 (2012). https://doi.org/10.1007/s11033-011-1122-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-011-1122-6