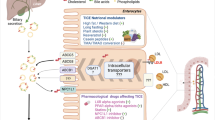
Abstract
Reverse cholesterol transport (RCT) and transintestinal cholesterol efflux (TICE) are two important pathways for body cholesterol elimination. We studied these pathways in an animal model of diabetes and obesity (ob/ob) where HDL function is compromised as a result of hyperglycemia, low-grade inflammation and oxidative stress. Co-treatment of ob/ob mice with PPAR-α (fenofibrate) and LXR (T0901317) agonists increased fecal cholesterol by 12-fold; PPAR-α and LXR agonists individually showed 2.6- and 4.0-fold fecal cholesterol excretion, respectively. We investigated the mechanism of synergistic efficacy of PPAR-α and LXR agonists in fecal cholesterol excretion. LXR agonist and the combination of PPAR-α and LXR agonists had greater HDL-C elevation. Ex vivo cholesterol efflux showed correlation with the fecal cholesterol excretion but was not sufficient to explain 12-fold increases in the fecal cholesterol in the co-treated mice. Therefore, we examined TICE to explain the 12-fold increases in the fecal cholesterol. A strong positive correlation of fecal cholesterol with ATP binding cassette transporter G5 (ABCG5) and G8 and a negative correlation with NPC1L1 was observed. ABCG5, G8 and NPC1L1 are involved in intestinal cholesterol absorption. The extent of influence of PPAR-α and LXR agonists on RCT and TICE was distinctly different. PPAR-α agonist increased fecal cholesterol primarily by influencing TICE, while LXR agonist influenced fecal cholesterol excretion via both RCT and TICE mechanisms. Synergistic efficacy on fecal cholesterol excretion following co-treatment with PPAR-α and LXR agonists occurred through a combination of RCT, TICE, and the key enzyme in bile synthesis, cholesterol 7-α hydroxylase (cyp7a1). These results suggest that cholesterol efflux, biliary cholesterol excretion, and TICE collectively contributed to the 12-fold increases in the fecal cholesterol excretion in ob/ob mice co-treated with PPAR-α and LXR agonists.








Similar content being viewed by others
Abbreviations
- CVD:
-
Cardiovascular disease
- CAD:
-
Coronary artery disease
- HDL-C:
-
High density lipoprotein cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
- RCT:
-
Reverse cholesterol transport
- ABCA1:
-
ATP binding cassette transporter A1
- ABCG5:
-
ATP binding cassette transporter G5
- ABCG8:
-
ATP binding cassette transporter G8
- LXR:
-
Liver x receptor
- PPAR:
-
Peroxisome proliferator activated receptor
- RXR:
-
Retinoid x receptor
- FAS:
-
Fatty acid synthase
- SCD1:
-
Stearoyl coA desaturase 1
- SREBP1c:
-
Sterol response element binding protein 1c
- Cyp7A1:
-
Cholesterol 7-alpha hydroxylase
- NPC1L1:
-
Niemann-Pick C1-Like 1
- Acox:
-
Acetyl-CoA oxidase
- SR-BI:
-
Scavenger receptor class B type 1
- ACAT:
-
Acyl-CoA cholesterol acyltransferase
- T1317:
-
T0901317
- TICE:
-
Transintestinal cholesterol efflux
- ELSD:
-
Evaporative light scattering detector
References
Linsel-Nitschke P, Tall AR (2005) HDL as a target in the treatment of atherosclerotic cardiovascular disease. Nat Rev Drug Discov 4:193–205. https://doi.org/10.1038/nrd1658
Srivastava RA, Srivastava N (2000) High density lipoprotein, apolipoprotein A-I, and coronary artery disease. Mol Cell Biochem 209:131–144
Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ (2011) Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med 364:127–135. https://doi.org/10.1056/NEJMoa1001689
Annema W, Tietge UJ (2012) Regulation of reverse cholesterol transport - a comprehensive appraisal of available animal studies. Nutr Metab (Lond) 9:25. https://doi.org/10.1186/1743-7075-9-25
Gordon DJ, Knoke J, Probstfield JL, Superko R, Tyroler HA (1986) High-density lipoprotein cholesterol and coronary heart disease in hypercholesterolemic men: the Lipid Research Clinics Coronary Primary Prevention Trial. Circulation 74:1217–1225
Investigators DAIS (2001) Effect of fenofibrate on progression of coronary-artery disease in type 2 diabetes: the Diabetes Atherosclerosis Intervention Study, a randomised study. Lancet 357:905–910
Frick MH, Elo O, Haapa K, Heinonen OP, Heinsalmi P, Helo P, Huttunen JK, Kaitaniemi P, Koskinen P, Manninen V et al (1987) Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med 317:1237–1245. https://doi.org/10.1056/nejm198711123172001
Rubins HB, Robins SJ, Collins D, Fye CL, Anderson JW, Elam MB, Faas FH, Linares E, Schaefer EJ, Schectman G, Wilt TJ, Wittes J (1999) Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med 341:410–418. https://doi.org/10.1056/nejm199908053410604
Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, Shear CL, Revkin JH, Buhr KA, Fisher MR, Tall AR, Brewer B (2007) Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 357:2109–2122. https://doi.org/10.1056/NEJMoa0706628
Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, Chaitman BR, Holme IM, Kallend D, Leiter LA, Leitersdorf E, McMurray JJ, Mundl H, Nicholls SJ, Shah PK, Tardif JC, Wright RS (2012) Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med 367:2089–2099. https://doi.org/10.1056/NEJMoa1206797
Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, Neeland IJ, Yuhanna IS, Rader DR, de Lemos JA, Shaul PW (2014) HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med 371:2383–2393. https://doi.org/10.1056/NEJMoa1409065
Srivastava RAK (2018) Dysfunctional HDL in diabetes mellitus and its role in the pathogenesis of cardiovascular disease. Mol Cell Biochem 440:167–187. https://doi.org/10.1007/s11010-017-3165-z
Oram JF, Lawn RM, Garvin MR, Wade DP (2000) ABCA1 is the cAMP-inducible apolipoprotein receptor that mediates cholesterol secretion from macrophages. J Biol Chem 275:34508–34511. https://doi.org/10.1074/jbc.M006738200
Kresanov P, Ahotupa M, Vasankari T, Kaikkonen J, Kahonen M, Lehtimaki T, Viikari J, Raitakari OT (2013) The associations of oxidized high-density lipoprotein lipids with risk factors for atherosclerosis: the Cardiovascular Risk in Young Finns Study. Free Radic Biol Med 65:1284–1290. https://doi.org/10.1016/j.freeradbiomed.2013.09.023
Morgantini C, Natali A, Boldrini B, Imaizumi S, Navab M, Fogelman AM, Ferrannini E, Reddy ST (2011) Anti-inflammatory and antioxidant properties of HDLs are impaired in type 2 diabetes. Diabetes 60:2617–2623. https://doi.org/10.2337/db11-0378
Passarelli M, Tang C, McDonald TO, O'Brien KD, Gerrity RG, Heinecke JW, Oram JF (2005) Advanced glycation end product precursors impair ABCA1-dependent cholesterol removal from cells. Diabetes 54:2198–2205
Patel DC, Albrecht C, Pavitt D, Paul V, Pourreyron C, Newman SP, Godsland IF, Valabhji J, Johnston DG (2011) Type 2 diabetes is associated with reduced ATP-binding cassette transporter A1 gene expression, protein and function. PLoS ONE 6:e22142. https://doi.org/10.1371/journal.pone.0022142
Klein R (1995) Hyperglycemia and microvascular and macrovascular disease in diabetes. Diabet Care 18:258–268
Srivastava N, Cefalu AB, Averna M, Srivastava RAK (2018) Lack of correlation of plasma HDL with fecal cholesterol and plasma cholesterol efflux capacity suggests importance of HDL functionality in attenuation of atherosclerosis. Front Physiol 9:1222. https://doi.org/10.3389/fphys.2018.01222
van der Veen JN, van Dijk TH, Vrins CL, van Meer H, Havinga R, Bijsterveld K, Tietge UJ, Groen AK, Kuipers F (2009) Activation of the liver X receptor stimulates trans-intestinal excretion of plasma cholesterol. J Biol Chem 284:19211–19219. https://doi.org/10.1074/jbc.M109.014860
Repa JJ, Berge KE, Pomajzl C, Richardson JA, Hobbs H, Mangelsdorf DJ (2002) Regulation of ATP-binding cassette sterol transporters ABCG5 and ABCG8 by the liver X receptors alpha and beta. J Biol Chem 277:18793–18800. https://doi.org/10.1074/jbc.M109927200
Jakulj L, Vissers MN, van Roomen CP, van der Veen JN, Vrins CL, Kunne C, Stellaard F, Kastelein JJ, Groen AK (2010) Ezetimibe stimulates faecal neutral sterol excretion depending on abcg8 function in mice. FEBS Lett 584:3625–3628. https://doi.org/10.1016/j.febslet.2010.07.035
van der Veen JN, Kruit JK, Havinga R, Baller JF, Chimini G, Lestavel S, Staels B, Groot PH, Groen AK, Kuipers F (2005) Reduced cholesterol absorption upon PPARdelta activation coincides with decreased intestinal expression of NPC1L1. J Lipid Res 46:526–534. https://doi.org/10.1194/jlr.M400400-JLR200
Temel RE, Lee RG, Kelley KL, Davis MA, Shah R, Sawyer JK, Wilson MD, Rudel LL (2005) Intestinal cholesterol absorption is substantially reduced in mice deficient in both ABCA1 and ACAT2. J Lipid Res 46:2423–2431. https://doi.org/10.1194/jlr.M500232-JLR200
Duong M, Uno K, Nankivell V, Bursill C, Nicholls SJ (2018) Induction of obesity impairs reverse cholesterol transport in ob/ob mice. PLoS ONE 13:e0202102. https://doi.org/10.1371/journal.pone.0202102
de Boer JF, Annema W, Schreurs M, van der Veen JN, van der Giet M, Nijstad N, Kuipers F, Tietge UJ (2012) Type I diabetes mellitus decreases in vivo macrophage-to-feces reverse cholesterol transport despite increased biliary sterol secretion in mice. J Lipid Res 53:348–357. https://doi.org/10.1194/jlr.M018671
Farbstein D, Levy AP (2012) HDL dysfunction in diabetes: causes and possible treatments. Expert Rev Cardiovasc Ther 10:353–361. https://doi.org/10.1586/erc.11.182
Bao LD, Li CQ, Peng R, Ren XH, Ma RL, Wang Y, Lv HJ (2015) Correlation between the decrease of cholesterol efflux from macrophages in patients with type II diabetes mellitus and down-regulated CYP7A1 expression. Genet Mol Res 14:8716–8724. https://doi.org/10.4238/2015.July.31.20
Srivastava RA, Srivastava N, Averna M, Lin RC, Korach KS, Lubahn DB, Schonfeld G (1997) Estrogen up-regulates apolipoprotein E (ApoE) gene expression by increasing ApoE mRNA in the translating pool via the estrogen receptor alpha-mediated pathway. J Biol Chem 272:33360–33366
Srivastava RA, Srivastava N, Averna M (2000) Dietary cholic acid lowers plasma levels of mouse and human apolipoprotein A-I primarily via a transcriptional mechanism. Eur J Biochem 267:4272–4280
Srivastava RA, He S, Newton RS (2011) Differential regulation of human apolipoprotein AI and high-density lipoprotein by fenofibrate in hapoAI and hapoAI-CIII-AIV transgenic mice. Biochim Biophys Acta 1811:76–83. https://doi.org/10.1016/j.bbalip.2010.11.004
Srivastava RA, Jahagirdar R, Azhar S, Sharma S, Bisgaier CL (2006) Peroxisome proliferator-activated receptor-alpha selective ligand reduces adiposity, improves insulin sensitivity and inhibits atherosclerosis in LDL receptor-deficient mice. Mol Cell Biochem 285:35–50. https://doi.org/10.1007/s11010-005-9053-y
Homan R, Anderson MK (1998) Rapid separation and quantitation of combined neutral and polar lipid classes by high-performance liquid chromatography and evaporative light-scattering mass detection. J Chromatogr B 708:21–26
Trieb M, Horvath A, Birner-Gruenberger R, Spindelboeck W, Stadlbauer V, Taschler U, Curcic S, Stauber RE, Holzer M, Pasterk L, Heinemann A, Marsche G (2016) Liver disease alters high-density lipoprotein composition, metabolism and function. Biochim Biophys Acta 1861:630–638. https://doi.org/10.1016/j.bbalip.2016.04.013
Srivastava RA, Srivastava N, Averna M, Cefalu AB, Schonfeld G (1999) Molecular bases of low production rates of apolipoprotein B-100 and truncated apoB-82 in a mutant HepG2 cell line generated by targeted modification of the apolipoprotein B gene. J Lipid Res 40:901–912
Srivastava RA (2009) Fenofibrate ameliorates diabetic and dyslipidemic profiles in KKAy mice partly via down-regulation of 11beta-HSD1, PEPCK and DGAT2. Comparison of PPARalpha, PPARgamma, and liver x receptor agonists. Eur J Pharmacol 607:258–263. https://doi.org/10.1016/j.ejphar.2009.02.024
Srivastava RA (2011) Evaluation of anti-atherosclerotic activities of PPAR-alpha, PPAR-gamma, and LXR agonists in hyperlipidemic atherosclerosis-susceptible F(1)B hamsters. Atherosclerosis 214:86–93. https://doi.org/10.1016/j.atherosclerosis.2010.10.033
Larrede S, Quinn CM, Jessup W, Frisdal E, Olivier M, Hsieh V, Kim MJ, Van Eck M, Couvert P, Carrie A, Giral P, Chapman MJ, Guerin M, Le Goff W (2009) Stimulation of cholesterol efflux by LXR agonists in cholesterol-loaded human macrophages is ABCA1-dependent but ABCG1-independent. Arterioscler Thromb Vasc Biol 29:1930–1936. https://doi.org/10.1161/atvbaha.109.194548
Schultz JR, Tu H, Luk A, Repa JJ, Medina JC, Li L, Schwendner S, Wang S, Thoolen M, Mangelsdorf DJ, Lustig KD, Shan B (2000) Role of LXRs in control of lipogenesis. Genes Dev 14:2831–2838
Chisholm JW, Hong J, Mills SA, Lawn RM (2003) The LXR ligand T0901317 induces severe lipogenesis in the db/db diabetic mouse. J Lipid Res 44:2039–2048. https://doi.org/10.1194/jlr.M300135-JLR200
Wiegman CH, Bandsma RH, Ouwens M, van der Sluijs FH, Havinga R, Boer T, Reijngoud DJ, Romijn JA, Kuipers F (2003) Hepatic VLDL production in ob/ob mice is not stimulated by massive de novo lipogenesis but is less sensitive to the suppressive effects of insulin. Diabetes 52:1081–1089
Jia L, Betters JL, Yu L (2011) Niemann-pick C1-like 1 (NPC1L1) protein in intestinal and hepatic cholesterol transport. Annu Rev Physiol 73:239–259. https://doi.org/10.1146/annurev-physiol-012110-142233
Altmann SW, Davis HR Jr, Zhu LJ, Yao X, Hoos LM, Tetzloff G, Iyer SP, Maguire M, Golovko A, Zeng M, Wang L, Murgolo N, Graziano MP (2004) Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science 303:1201–1204. https://doi.org/10.1126/science.1093131
Yamamoto H, Yamanashi Y, Takada T, Mu S, Tanaka Y, Komine T, Suzuki H (2019) Hepatic expression of niemann-pick C1-like 1, a cholesterol reabsorber from bile, exacerbates western diet-induced atherosclerosis in LDL receptor mutant mice. Mol Pharmacol 96:47–55. https://doi.org/10.1124/mol.119.115840
Laffitte BA, Chao LC, Li J, Walczak R, Hummasti S, Joseph SB, Castrillo A, Wilpitz DC, Mangelsdorf DJ, Collins JL, Saez E, Tontonoz P (2003) Activation of liver X receptor improves glucose tolerance through coordinate regulation of glucose metabolism in liver and adipose tissue. Proc Natl Acad Sci USA 100:5419–5424. https://doi.org/10.1073/pnas.0830671100
Knauf C, Rieusset J, Foretz M, Cani PD, Uldry M, Hosokawa M, Martinez E, Bringart M, Waget A, Kersten S, Desvergne B, Gremlich S, Wahli W, Seydoux J, Delzenne NM, Thorens B, Burcelin R (2006) Peroxisome proliferator-activated receptor-alpha-null mice have increased white adipose tissue glucose utilization, GLUT4, and fat mass: role in liver and brain. Endocrinology 147:4067–4078. https://doi.org/10.1210/en.2005-1536
Sahoo D, Trischuk TC, Chan T, Drover VA, Ho S, Chimini G, Agellon LB, Agnihotri R, Francis GA, Lehner R (2004) ABCA1-dependent lipid efflux to apolipoprotein A-I mediates HDL particle formation and decreases VLDL secretion from murine hepatocytes. J Lipid Res 45:1122–1131. https://doi.org/10.1194/jlr.M300529-JLR200
Wroblewska M (2011) The origin and metabolism of a nascent pre-beta high density lipoprotein involved in cellular cholesterol efflux. Acta Biochim Pol 58:275–285
Altemus JB, Patel SB, Sehayek E (2014) Liver-specific induction of Abcg5 and Abcg8 stimulates reverse cholesterol transport in response to ezetimibe treatment. Metabolism 63:1334–1341. https://doi.org/10.1016/j.metabol.2014.06.014
Briand F, Naik SU, Fuki I, Millar JS, Macphee C, Walker M, Billheimer J, Rothblat G, Rader DJ (2009) Both the peroxisome proliferator-activated receptor delta agonist, GW0742, and ezetimibe promote reverse cholesterol transport in mice by reducing intestinal reabsorption of HDL-derived cholesterol. Clin Transl Sci 2:127–133
Hozoji-Inada M, Munehira Y, Nagao K, Kioka N, Ueda K (2011) Liver X receptor beta (LXRbeta) interacts directly with ATP-binding cassette A1 (ABCA1) to promote high density lipoprotein formation during acute cholesterol accumulation. J Biol Chem 286:20117–20124. https://doi.org/10.1074/jbc.M111.235846
Berthou L, Duverger N, Emmanuel F, Langouet S, Auwerx J, Guillouzo A, Fruchart JC, Rubin E, Denefle P, Staels B, Branellec D (1996) Opposite regulation of human versus mouse apolipoprotein A-I by fibrates in human apolipoprotein A-I transgenic mice. J Clin Invest 97:2408–2416. https://doi.org/10.1172/jci118687
Vu-Dac N, Chopin-Delannoy S, Gervois P, Bonnelye E, Martin G, Fruchart JC, Laudet V, Staels B (1998) The nuclear receptors peroxisome proliferator-activated receptor alpha and Rev-erbalpha mediate the species-specific regulation of apolipoprotein A-I expression by fibrates. J Biol Chem 273:25713–25720
Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD, Jacobs DR Jr, Bangdiwala S, Tyroler HA (1989) High-density lipoprotein cholesterol and cardiovascular disease Four Prospect American studies. Circulation 79:8–15
Goldbourt U, Yaari S, Medalie JH (1997) Isolated low HDL cholesterol as a risk factor for coronary heart disease mortality. A 21-year follow-up of 8000 men. Arterioscler Thromb Vasc Biol 17:107–113
Kosmas CE, DeJesus E, Rosario D, Vittorio TJ (2016) CETP inhibition: past failures and future hopes. Clin Med Insights Cardiol 10:37–42. https://doi.org/10.4137/cmc.s32667
Chen Z, Wang SP, Krsmanovic ML, Castro-Perez J, Gagen K, Mendoza V, Rosa R, Shah V, He T, Stout SJ, Geoghagen NS, Lee SH, McLaren DG, Wang L, Roddy TP, Plump AS, Hubbard BK, Sinz CJ, Johns DG (2012) Small molecule activation of lecithin cholesterol acyltransferase modulates lipoprotein metabolism in mice and hamsters. Metabolism 61:470–481. https://doi.org/10.1016/j.metabol.2011.08.006
Di Bartolo BA, Scherer DJ, Nicholls SJ (2016) Inducing apolipoprotein A-I synthesis to reduce cardiovascular risk: from ASSERT to SUSTAIN and beyond. Arch Med Sci 12:1302–1307. https://doi.org/10.5114/aoms.2016.62906
Balder JW, Staels B, Kuivenhoven JA (2013) Pharmacological interventions in human HDL metabolism. Curr Opin Lipidol 24:500–509. https://doi.org/10.1097/mol.0000000000000018
Jaleel A, Henderson GC, Madden BJ, Klaus KA, Morse DM, Gopala S, Nair KS (2010) Identification of de novo synthesized and relatively older proteins: accelerated oxidative damage to de novo synthesized apolipoprotein A-1 in type 1 diabetes. Diabetes 59:2366–2374. https://doi.org/10.2337/db10-0371
Okuda LS, Castilho G, Rocco DD, Nakandakare ER, Catanozi S, Passarelli M (2012) Advanced glycated albumin impairs HDL anti-inflammatory activity and primes macrophages for inflammatory response that reduces reverse cholesterol transport. Biochim Biophys Acta 1821:1485–1492. https://doi.org/10.1016/j.bbalip.2012.08.011
Schwartz K, Lawn RM, Wade DP (2000) ABC1 gene expression and ApoA-I-mediated cholesterol efflux are regulated by LXR. Biochem Biophys Res Commun 274:794–802. https://doi.org/10.1006/bbrc.2000.3243
van der Velde AE, Vrins CL, van den Oever K, Kunne C, Oude Elferink RP, Kuipers F, Groen AK (2007) Direct intestinal cholesterol secretion contributes significantly to total fecal neutral sterol excretion in mice. Gastroenterology 133:967–975. https://doi.org/10.1053/j.gastro.2007.06.019
Valasek MA, Clarke SL, Repa JJ (2007) Fenofibrate reduces intestinal cholesterol absorption via PPARalpha-dependent modulation of NPC1L1 expression in mouse. J Lipid Res 48:2725–2735. https://doi.org/10.1194/jlr.M700345-JLR200
Vrins CL, Ottenhoff R, van den Oever K, de Waart DR, Kruyt JK, Zhao Y, van Berkel TJ, Havekes LM, Aerts JM, van Eck M, Rensen PC, Groen AK (2012) Trans-intestinal cholesterol efflux is not mediated through high density lipoprotein. J Lipid Res 53:2017–2023. https://doi.org/10.1194/jlr.M022194
Srivastava RA, Baumann D, Schonfeld G (1993) In vivo regulation of low-density lipoprotein receptors by estrogen differs at the post-transcriptional level in rat and mouse. Eur J Biochem 216:527–538
Van Eck M, Pennings M, Hoekstra M, Out R, Van Berkel TJ (2005) Scavenger receptor BI and ATP-binding cassette transporter A1 in reverse cholesterol transport and atherosclerosis. Curr Opin Lipidol 16:307–315
Kennedy MA, Barrera GC, Nakamura K, Baldan A, Tarr P, Fishbein MC, Frank J, Francone OL, Edwards PA (2005) ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab 1:121–131. https://doi.org/10.1016/j.cmet.2005.01.002
Kane JP, Malloy MJ (2012) Prebeta-1 HDL and coronary heart disease. Curr Opin Lipidol 23:367–371. https://doi.org/10.1097/MOL.0b013e328353eef1
Mardones P, Pilon A, Bouly M, Duran D, Nishimoto T, Arai H, Kozarsky KF, Altayo M, Miquel JF, Luc G, Clavey V, Staels B, Rigotti A (2003) Fibrates down-regulate hepatic scavenger receptor class B type I protein expression in mice. J Biol Chem 278:7884–7890. https://doi.org/10.1074/jbc.M211627200
Chinetti G, Gbaguidi FG, Griglio S, Mallat Z, Antonucci M, Poulain P, Chapman J, Fruchart JC, Tedgui A, Najib-Fruchart J, Staels B (2000) CLA-1/SR-BI is expressed in atherosclerotic lesion macrophages and regulated by activators of peroxisome proliferator-activated receptors. Circulation 101:2411–2417
Li T, Matozel M, Boehme S, Kong B, Nilsson LM, Guo G, Ellis E, Chiang JY (2011) Overexpression of cholesterol 7alpha-hydroxylase promotes hepatic bile acid synthesis and secretion and maintains cholesterol homeostasis. Hepatology 53:996–1006. https://doi.org/10.1002/hep.24107
Duval C, Touche V, Tailleux A, Fruchart JC, Fievet C, Clavey V, Staels B, Lestavel S (2006) Niemann-Pick C1 like 1 gene expression is down-regulated by LXR activators in the intestine. Biochem Biophys Res Commun 340:1259–1263. https://doi.org/10.1016/j.bbrc.2005.12.137
Jia L, Ma Y, Rong S, Betters JL, **e P, Chung S, Wang N, Tang W, Yu L (2010) Niemann-Pick C1-Like 1 deletion in mice prevents high-fat diet-induced fatty liver by reducing lipogenesis. J Lipid Res 51:3135–3144. https://doi.org/10.1194/jlr.M006353
Jakulj L, van Dijk TH, de Boer JF, Kootte RS, Schonewille M, Paalvast Y, Boer T, Bloks VW, Boverhof R, Nieuwdorp M, Beuers UH, Stroes ES, Groen AK (2016) Transintestinal cholesterol transport is active in mice and humans and controls ezetimibe-induced fecal neutral sterol excretion. Cell Metab 24:783–794. https://doi.org/10.1016/j.cmet.2016.10.001
Traldi P, Castilho G, Sartori CH, Machado-Lima A, Nakandakare ER, Correa-Giannella ML, Roverso M, Porcu S, Lapolla A, Passarelli M (2015) Glycated human serum albumin isolated from poorly controlled diabetic patients impairs cholesterol efflux from macrophages: an investigation by mass spectrometry. Eur J Mass Spectrom (Chichester, Eng) 21:233–244. https://doi.org/10.1255/ejms.1322
Srivastava R, Cefalu, AB, Davide, A and Averna, MR (2013) A Combination of Metformin, Quercetin, and Curcumin Restores HDL Function and Improves Atherosclerosis Burden in LDLr−/−/ob.ob leptin−/− and LDLr−/− Mice by attenuating Insulin Resistance, Hyperglycemia, and Low-grade Inflammation. ATVB Scientific Session, Abstract
Kusakabe T, Tanioka H, Ebihara K, Hirata M, Miyamoto L, Miyanaga F, Hige H, Aotani D, Fujisawa T, Masuzaki H, Hosoda K, Nakao K (2009) Beneficial effects of leptin on glycaemic and lipid control in a mouse model of type 2 diabetes with increased adiposity induced by streptozotocin and a high-fat diet. Diabetologia 52:675–683. https://doi.org/10.1007/s00125-009-1258-2
Shiomi Y, Yamauchi T, Iwabu M, Okada-Iwabu M, Nakayama R, Orikawa Y, Yoshioka Y, Tanaka K, Ueki K, Kadowaki T (2015) A novel peroxisome proliferator-activated receptor (PPAR)α agonist and PPARγ antagonist, Z-551, ameliorates high-fat diet-induced obesity and metabolic disorders in mice. J Biol Chem 290:14567–14581. https://doi.org/10.1074/jbc.M114.622191
Suzuki A, Okamoto S, Lee S, Saito K, Shiuchi T, Minokoshi Y (2007) Leptin stimulates fatty acid oxidation and peroxisome proliferator-activated receptor alpha gene expression in mouse C2C12 myoblasts by changing the subcellular localization of the alpha2 form of AMP-activated protein kinase. Mol Cell Biol 27:4317–4327. https://doi.org/10.1128/mcb.02222-06
Korach-André M, Archer A, Barros RP, Parini P, Gustafsson J (2011) Both liver-X receptor (LXR) isoforms control energy expenditure by regulating brown adipose tissue activity. Proc Natl Acad Sci USA 108:403–408. https://doi.org/10.1073/pnas.1017884108
Author information
Authors and Affiliations
Contributions
Rai Ajit K Srivastava designed the study, evaluated data from the studies and contributed to the interpretation and analyses of data as well as writing of the manuscript. Angelo Cefalu performed some studies and wrote part of the manuscript, and Nishtha Srivastava contributed to the animal studies. Maurizio Averna contributed to the review and interpretation of the data and wrote part of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Rai Ajit K Srivastava works as an independent consultant to Biotech companies. All authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Srivastava, R.A.K., Cefalu, A.B., Srivastava, N.S. et al. NPC1L1 and ABCG5/8 induction explain synergistic fecal cholesterol excretion in ob/ob mice co-treated with PPAR-α and LXR agonists. Mol Cell Biochem 473, 247–262 (2020). https://doi.org/10.1007/s11010-020-03826-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03826-3