Abstract
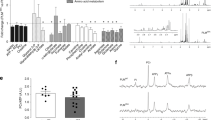
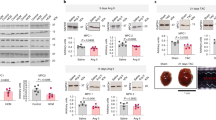
Cardiac hypertrophy is an independent risk factor in the development of heart failure. However, the cellular mechanisms underlying the transition from compensated hypertrophy to heart failure are incompletely understood. The aim of this study was to investigate changes in myocardial substrate utilisation and function in pressure-overload hypertrophy (using 13C NMR spectroscopy) in parallel with alterations in the expression pattern of genes involved in cardiac fatty acid and glucose uptake and oxidation. Left ventricular hypertrophy was induced surgically in Sprague–Dawley rats by inter-renal aortic constriction. Nine weeks later, hearts were perfused in the isovolumic mode with a physiological mixture of substrates including 5 mM 1-13C glucose, 1 mM 3-13C lactate, 0.1 mM U-13C pyruvate and 0.3 mM U-13C palmitate and cardiac function monitored simultaneously. Real-time PCR was used to determine mRNA levels of PPARα and PPARα-regulated metabolic enzymes. Results showed that at the stage of compensated hypertrophy, fatty acid oxidation (FAO) and expression of genes involved in FAO were markedly reduced, whilst pyruvate oxidation was enhanced, highlighting the fact that metabolic remodelling is an early event in the development of cardiac hypertrophy.


Similar content being viewed by others
Abbreviations
- ATP:
-
Adenosine triphosphate
- MCAD:
-
Medium-chain acyl-CoA dehydrogenase
- NMR:
-
Nuclear magnetic resonance
- PCR:
-
Polymerase chain reaction
- PDH:
-
Pyruvate dehydrogenase
- PPARα:
-
Peroxisome proliferator-activated receptor-α
- PGC1:
-
Peroxisome-proliferator-activated receptor-γ co-activator 1
References
Seymour AM (2003) Mitochondrial function—a limiting factor in heart failure? In: Dhalla NS, Rupp H (eds) Pathophysiology of cardiovascular disease, 1st edn. Kluwer academic publishers, Boston, pp 23–33
Frohlich ED, Apstein C, Chobanian AV, Devereux RB, Dustan HP, Dzau V, Fauad-Tarazi F, Horan MJ, Marcus M, Massie B (1992) The heart in hypertension. N Engl J Med 327:998–1008
Lehman JJ, Kelly DP (2002) Gene regulatory mechanisms governing energy metabolism during cardiac hypertrophic growth. Heart Fail Rev 7:175–185
Hawkins NM, Wang D, McMurray JJ, Pfeffer MA, Swedberg K, Granger CB, Yusuf S, Pocock SJ, Ostergren J, Michelson EL, Dunn FG (2007) Prevalence and prognostic implications of electrocardiographic left ventricular hypertrophy in heart failure: evidence from the CHARM programme. Heart 93:59–64
Thom T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, Zheng ZJ, Flegal K, O’Donnell C, Kittner S, Lloyd-Jones D, Goff DC Jr, Hong Y, Adams R, Friday G, Furie K, Gorelick P, Kissela B, Marler J, Meigs J, Roger V, Sidney S, Sorlie P, Steinberger J, Wasserthiel-Smoller S, Wilson M, Wolf P (2006) Heart disease and stroke statistics–2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 113:e85–e151
van Bilsen M, Smeets PJ, Gilde AJ, van der Vusse GJ (2004) Metabolic remodelling of the failing heart: the cardiac burn-out syndrome? Cardiovasc Res 61:218–226
Gerdes AM (2002) Cardiac myocyte remodeling in hypertrophy and progression to failure. J Card Fail 8:S264–S268
Burgess ML, Buggy J, Price RL, Abel FL, Terracio L, Samarel AM, Borg TK (1996) Exercise- and hypertension-induced collagen changes are related to left ventricular function in rat hearts. Am J Physiol 270:H151–H159
Bers DM (2006) Altered cardiac myocyte Ca regulation in heart failure. Physiology (Bethesda) 21:380–387
Allard MF, Schonekess BO, Henning SL, English DR, Lopaschuk GD (1994) Contribution of oxidative metabolism and glycolysis to ATP production in hypertrophied hearts. Am J Physiol 267:H742–H750
Christe ME, Rodgers RL (1994) Altered glucose and fatty acid oxidation in hearts of the spontaneously hypertensive rat. J Mol Cell Cardiol 26:1371–1375
el Alaoui-Talibi Z, Guendouz A, Moravec M, Moravec J (1997) Control of oxidative metabolism in volume-overloaded rat hearts: effect of propionyl-L-carnitine. Am J Physiol 272:H1615–H1624
Stanley WC, Recchia FA, Lopaschuk GD (2005) Myocardial substrate metabolism in the normal and failing heart. Physiol Rev 85:1093–1129
Lloyd S, Brocks C, Chatham JC (2003) Differential modulation of glucose, lactate, and pyruvate oxidation by insulin and dichloroacetate in the rat heart. Am J Physiol Heart Circ Physiol 285:H163–H172
Mallet RT, Sun J (1999) Mitochondrial metabolism of pyruvate is required for its enhancement of cardiac function and energetics. Cardiovasc Res 42:149–161
Sack MN, Rader TA, Park S, Bastin J, McCune SA, Kelly DP (1996) Fatty acid oxidation enzyme gene expression is downregulated in the failing heart. Circulation 94:2837–2842
Razeghi P, Young ME, Alcorn JL, Moravec CS, Frazier OH, Taegtmeyer H (2001) Metabolic gene expression in fetal and failing human heart. Circulation 104:2923–2931
Rosenblatt-Velin N, Montessuit C, Papageorgiou I, Terrand J, Lerch R (2001) Postinfarction heart failure in rats is associated with upregulation of GLUT-1 and downregulation of genes of fatty acid metabolism. Cardiovasc Res 52:407–416
Boateng S, Seymour AM, Dunn M, Yacoub M, Boheler K (1997) Inhibition of endogenous cardiac phosphatase activity and measurement of sarcoplasmic reticulum calcium uptake: a possible role of phospholamban phosphorylation in the hypertrophied myocardium. Biochem Biophys Res Commun 239:701–705
Yin FC, Spurgeon HA, Rakusan K, Weisfeldt ML, Lakatta EG (1982) Use of tibial length to quantify cardiac hypertrophy: application in the aging rat. Am J Physiol 243:H941–H947
Sample J, Cleland JG, Seymour AM (2006) Metabolic remodeling in the aging heart. J Mol Cell Cardiol 40:56–63
How OJ, Aasum E, Kunnathu S, Severson DL, Myhre ES, Larsen TS (2005) Influence of substrate supply on cardiac efficiency, as measured by pressure-volume analysis in ex vivo mouse hearts. Am J Physiol Heart Circ Physiol 288:H2979–H2985
Seymour AM, Eldar H, Radda GK (1990) Hyperthyroidism results in increased glycolytic capacity in the rat heart. A 31P-NMR study. Biochim Biophys Acta 1055:107–116
Malloy CR, Sherry AD, Jeffrey FM (1990) Analysis of tricarboxylic acid cycle of the heart using 13C isotope isomers. Am J Physiol 259:H987–H995
Lloyd SG, Wang P, Zeng H, Chatham JC (2004) Impact of low-flow ischemia on substrate oxidation and glycolysis in the isolated perfused rat heart. Am J Physiol Heart Circ Physiol 287:H351–H362
Malloy CR, Jones JG, Jeffrey FM, Jessen ME, Sherry AD (1996) Contribution of various substrates to total citric acid cycle flux and anaplerosis as determined by 13C isotopomer analysis and O2 consumption in the heart. Magma 4:35–46
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Richardson S (2002) Studies of ischaemia and reperfusion in cardiac hypertrophy, PhD thesis. University of Hull, Hull, p 253
Lehman TC, Hale DE, Bhala A, Thorpe C (1990) An acyl-coenzyme A dehydrogenase assay utilizing the ferricenium ion. Anal Biochem 186:280–284
Seymour AM, Chatham JC (1997) The effects of hypertrophy and diabetes on cardiac pyruvate dehydrogenase activity. J Mol Cell Cardiol 29:2771–2778
Trinder P (1969) Determination of blood glucose using an oxidase-peroxidase system with a non-carcinogenic chromogen. J Clin Pathol 22:158–161
Sambrook J, Russel DW (2001) Extraction, purification and analysis of mRNA from eukaryotic cells. 7.3–7.25
Boheler KR, Volkova M, Morrell C, Garg R, Zhu Y, Margulies K, Seymour AM Lakatta EG (2003) Sex- and age-dependent human transcriptome variability: implications for chronic heart failure. Proc Natl Acad Sci U S A 100:2754–2759
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408
Boateng SY, Seymour AM, Bhutta NS, Dunn MJ, Yacoub MH, Boheler KR (1998) Sub-antihypertensive doses of ramipril normalize sarcoplasmic reticulum calcium ATPase expression and function following cardiac hypertrophy in rats. J Mol Cell Cardiol 30:2683–2694
Bonen A, Luiken JJ, Arumugam Y, Glatz JF, Tandon NN (2000) Acute regulation of fatty acid uptake involves the cellular redistribution of fatty acid translocase. J Biol Chem 275:14501–14508
Santalucia T, Camps M, Castello A, Munoz P, Nuel A, Testar X, Palacin M, Zorzano A (1992) Developmental regulation of GLUT-1 (erythroid/Hep G2) and GLUT-4 (muscle/fat) glucose transporter expression in rat heart, skeletal muscle, and brown adipose tissue. Endocrinology 130:837–846
Morgan EE, Chandler MP, Young ME, McElfresh TA, Kung TA, Rennison JH, Tserng KY, Hoit BD, Stanley WC (2006) Dissociation between gene and protein expression of metabolic enzymes in a rodent model of heart failure. Eur J Heart Fail 8:687–693
Allard MF, Henning SL, Wambolt RB, Granleese SR, English DR, Lopaschuk GD (1997) Glycogen metabolism in the aerobic hypertrophied rat heart. Circulation 96:676–682
Mjos OD (1971) Effect of free fatty acids on myocardial function and oxygen consumption in intact dogs. J Clin Invest 50:1386–1389
Burkhoff D, Weiss RG, Schulman SP, Kalil-Filho R, Wannenburg T, Gerstenblith G (1991) Influence of metabolic substrate on rat heart function and metabolism at different coronary flows. Am J Physiol 261:H741–H750
Houser SR, Piacentino V, Weisser J (2000) Abnormalities of calcium cycling in the hypertrophied and failing heart. J Mol Cell Cardiol 32:1595–1607
Vincent G, Khairallah M, Bouchard B, Des Rosiers C (2003) Metabolic phenoty** of the diseased rat heart using 13C-substrates and ex vivo perfusion in the working mode. Mol Cell Biochem 242:89–99
Labarthe F, Khairallah M, Bouchard B, Stanley WC, Des Rosiers C (2005) Fatty acid oxidation and its impact on response of spontaneously hypertensive rat hearts to an adrenergic stress: benefits of a medium-chain fatty acid. Am J Physiol Heart Circ Physiol 288:H1425–H1436
Borst P, Loos JA, Christ EJ, Slater EC (1962) Uncoupling activity of long-chain fatty acids. Biochim Biophys Acta 62:509–518
Garlick PB, Mashiter GD, Di Marzo V, Tippins JR, Morris HR, Maisey MN (1989) The synthesis, release and action of leukotrienes in the isolated, unstimulated, buffer-perfused rat heart. J Mol Cell Cardiol 21:1101–1110
Drake AJ, Haines JR, Noble MI (1980) Preferential uptake of lactate by the normal myocardium in dogs. Cardiovasc Res 14:65–72
Goodwin GW, Arteaga JR, Taegtmeyer H (1995) Glycogen turnover in the isolated working rat heart. J Biol Chem 270:9234–9240
Young ME, Patil S, Ying J, Depre C, Ahuja HS, Shipley GL, Stepkowski SM, Davies PJ, Taegtmeyer H (2001) Uncoupling protein 3 transcription is regulated by peroxisome proliferator-activated receptor (alpha) in the adult rodent heart. Faseb J 15:833–845
Degens H, de Brouwer KF, Gilde AJ, Lindhout M, Willemsen PH, Janssen BJ, van der Vusse GJ, van Bilsen M (2006) Cardiac fatty acid metabolism is preserved in the compensated hypertrophic rat heart. Basic Res Cardiol 101:17–26
Djouadi F, Weinheimer CJ, Saffitz JE, Pitchford C, Bastin J, Gonzalez FJ, Kelly DP (1998) A gender-related defect in lipid metabolism and glucose homeostasis in peroxisome proliferator-activated receptor alpha-deficient mice. J Clin Invest 102:1083–1091
Sack MN, Disch DL, Rockman HA, Kelly DP (1997) A role for Sp and nuclear receptor transcription factors in a cardiac hypertrophic growth program. Proc Natl Acad Sci U S A 94:6438–6443
Young ME, Laws FA, Goodwin GW, Taegtmeyer H (2001) Reactivation of peroxisome proliferator-activated receptor alpha is associated with contractile dysfunction in hypertrophied rat heart. J Biol Chem 276:44390–44395
Young ME (2006) The circadian clock within the heart: potential influence on myocardial gene expression, metabolism and function. Am J Physiol Heart Circ Physiol 290:H1–H16
Boss O, Hagen T, Lowell BB (2000) Uncoupling proteins 2 and 3: potential regulators of mitochondrial energy metabolism. Diabetes 49:143–156
Boehm EA, Jones BE, Radda GK, Veech RL, Clarke K (2001) Increased uncoupling proteins and decreased efficiency in palmitate-perfused hyperthyroid rat heart. Am J Physiol Heart Circ Physiol 280:H977–H983
Strom CC, Aplin M, Ploug T, Christoffersen TE, Langfort J, Viese M, Galbo H, Haunso S, Sheikh SP (2005) Expression profiling reveals differences in metabolic gene expression between exercise-induced cardiac effects and maladaptive cardiac hypertrophy. Febs J 272:2684–2695
Paternostro G, Clarke K, Heath J, Seymour AM, Radda GK (1995) Decreased GLUT-4 mRNA content and insulin-sensitive deoxyglucose uptake show insulin resistance in the hypertensive rat heart. Cardiovasc Res 30:205–211
Acknowledgements
Ashwin Akki was a recipient of the Graduate Teaching Assistantship from the University of Hull. We thank Mrs. Jenny Foster and Mrs. Kath Bulmer for excellent technical support, Mr. Denys Bilko for technical advice with Real-time PCR and Mr. K.Y. Lee for help with Western blotting. Thanks also go to Dr. Jessica Sample for constructive discussions and critical review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Akki, A., Smith, K. & Seymour, AM.L. Compensated cardiac hypertrophy is characterised by a decline in palmitate oxidation. Mol Cell Biochem 311, 215–224 (2008). https://doi.org/10.1007/s11010-008-9711-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-008-9711-y