Abstract
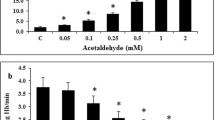
Alcohol intake is associated with numerous degenerative disorders, and the detrimental effects of alcohol may be due to its influence on plasma membrane and cellular transport systems. The aim of the present study was to compare in vitro and in vivo effects of ethanol on rabbit erythrocyte ATPase activities and correlate them with ethanol-induced oxidative stress. Age-matched male rabbits were given 5% ethanol in 2% sucrose solution, for 6 weeks ad libitum; control animals were given tap water. Daily intake of ethanol was 5 g/kg body weight; this experimental regimen resulted in an average serum ethanol concentration of 16.77 ± 2.00 mM/l. After this period, blood was collected, serum ethanol concentration was determined and erythrocyte membranes were prepared according to the method of Post et al. Activities of Na+/K+- and Mg2+-ATPases were determined. Thiobarbituric acid-reactive substance (TBARS) assay was used to detect levels of lipid peroxidation, a major indicator of oxidative stress. In vitro ethanol inhibits both Na+/K+-ATPase and Mg2+-ATPase, but Na+/K+-ATPase is more sensitive to the ethanol-induced inhibition. Increasing concentration of ethanol increased TBARS production, but significant difference was attained only at 5 and 12.5 mM of ethanol. Chronic ethanol consumption induced significant increase in Na+/K+- and Mg2+-ATPase activity, and TBARS production. Our results suggest that increased ATPase activity induced by chronic ethanol consumption is due to oxidative, induced modification of membrane phospholipids and proteins, which are responsible for inhibition of ATPase activity. Increased production of TBARS induced by in vitro exposure to ethanol is not the only factor that influences ATPases activity. Further research is needed to elucidate this relationship.





Similar content being viewed by others
References
Vasiletes SA, Schwarz W (1993) Structure-function relationship of cation binding in Na+/K+-ATPase. Biochem Biophys Acta 1154:201–222
Rodrigez LA, Arnaiz G, Pena C (1995) Characterization of Na+/K+-ATPase. Neurochem Int 27:319–327
Schuurmans Stekhoven FMAH (1998) E31-K352, the minimal cation binding moiety of Na+/K+-ATPase. Biochem Biophys Res Commun 245:366–369
Sanui H, Rubin H (1982) The role of magnesium in cell proliferation and transformation. In: Boynton AL, MacKeehan WL, Winitfield JP (eds) Ions, cell proliferation and cancer. Academic press, New York, pp 517–537
Tanaka T, Inagake C, Kungy Y, Takaori S (1987) Solubilisation and separation of ethachrynic acid (EA) highly sensitive and EA less sensitive Mg2+-ATPase at rat brain. Jpn J Pharmacol 45:205–211
Vujisic Lj, Krstic D, Vucetic J (2000) Chemical aspect influence of cobalt ions on ATPase activity. J Serb Chem Soc 65:507–515
Vasic V, Jovanovic D, Krstic D, Nikezic G, Horvat A, Vujisic Lj, Nedeljkovic N (1999) Prevention and recovery of CuSO4-induced inhibition of Na+/K+-ATPase in rat brain synaptosomes by EDTA. Toxicol Lett 110:95–104
Sadrzadeh SM, Price P, Nanji AA (1994) Ethanol-induced changes in membrane ATPases: inhibition by iron chelation. Biochem Pharmacol 47:745–747
Adachi J, Asano M, Ueno Y, Niemelä O, Ohlendieck K, Peters T, Preedy VR (2003) Alcoholic muscle disease and biomembrane perturbations. J Nutr Biochem 14:616–625
Johnson JH, Crider BP (1989) Increases in Na+/K+-ATPase activity of erythrocytes and skeletal muscle after chronic ethanol consumption: evidence for reduced efficiency of the enzyme. Proc Natl Acad Sci U S A 86:7857–7860
Foley TD, Rhoads DE (1994) Stimulation of synaptosomal Na+, K+-ATPase by ethanol: possible involvement of an isozyme-specific inhibitor of Na+, K+-ATPase. Brain Res 653:167–172
Zamai TN, Titova NM, Zamai AS, Usoltseva OS, Yulenkova OV, Shumkova DA (2002) Effect of alcoholic intoxication on water content and activity of Na, K-ATPase and Ca-ATPase in rat brain. Bull Exp Biol Med 134:541–543
Rodrigo R, Thielemann L (1997) Effects of chronic and acute ethanol exposure on renal (Na+K)-ATPase in the rat. Gen Pharmacol 29:719–723
Rodrigo R, Thielemann L, Orellana M (1998) Acute and chronic effect of ethanol on (Na+K)-ATPase activity and cyclic AMP response to vasopressin in rat papillary collecting duct cells. Gen Pharmacol 30:663–667
Gabbianelli R, Cifani C, Massi M, Polidori C, Falcioni G (2007) Oxidative damage in rat erythrocyte membranes following ethanol intake: effect of ethyl pyruvate. Chem Biol Interact 169(2):122–131
Dobrota D, Matejovicova M, Kurella EG (1999) Na+/K+ ATPase under oxidative stress: molecular mechanisms of injury. Cell Mol Neurobiol 19:141–149
Lefevre G, Beljean-Leymarie M, Beyerle F, Bonnefont-Rousselot D, Cristol JP, Therond P, Torreilles J (1998) Evaluation of lipid peroxidation by measuring thiobarbituric acid reactive substances. Ann Biol Clin (Paris) 56:305–319
Saito M, Broderick G, Levin R (1994) Effect of chronic ethanol consumption on the pharmacological response of the rabbit corpus cavernosum. Pharmacology 49:386–391
Post RI, Merit CR, Kosolving CR, Albbright CD (1960) Membrane adenosine triphosphatase as a participant in the active transport of sodium and potassium in the human erythrocyte. J Biol Chem 235:1796–1802
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 198:255–270
El Demrdash FM (2007) Lambda-cyhalothrin-induced changes in oxidative stress biomarkers in rabbit erythrocytes and alleviation effect of some antioxidants. Toxicol In Vitro 21:392–397
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Foley TD, Rhoads DE (1994) Stimulation of synaptosomal Na+, K+-ATPase by ethanol: possible involvement of an isozyme-specific inhibitor of Na+, K+-ATPase. Brain Res 653:167–172
Swann AC (1990) Ethanol inhibition of active 86Rb+-transport: evidence for enhancement by sodium or calcium influx. J Pharmacol Exp Ther 254:864–871
Syapin PJ, Chen J, Alkana RL (1985) Effect of norepinephrine on inhibition of mouse brain Na+/K+-stimulated, Mg++-dependent and Ca++-dependent ATPase activities by ethanol. Alcohol 2:145–148
Ferguson ER, Blachley JD, Carter NW, Knochel JP (1984) Derangements of muscle composition, ion transport, and oxygen consumption on chronically alcoholic dogs. Am J Physiol Renal Physiol 246:F700–F709
Li W, Zheng T, Babu AN, Altura B, Gupta RK, Altura B (2001) Importance of magnesium ions in development of tolerance to ethanol: studies on cultured cerebral vascular smooth muscle cells, type-2 astrocytes and intact rat brain. Brain Res Bull 56:153–158
Katz AM (1982) Effects of ethanol on ion transport in muscle membranes. Fed Proc 41:2456–2459
Dianzani MU (1985) Lipid peroxidation in ethanol poisoning: a critical reconsideration. Alcohol Alcohol 20(2):161–173
Ishii H, Kurose I, Kato S (1997) Pathogenesis of alcoholic liver disease with particular emphasis on oxidative stress. J Gastroenterol Hepatol 12:S272–S282
Akkuş I, Gültekin F, Aköz M, Çağlayan O, Bahçaci S, Can UG, Ay M, Gürel A (1997) Effect of moderate alcohol intake on lipid peroxidation in plasma, erythrocyte and leukocyte and on some antioxidant enzymes. Clin Chim Acta 266:141–147
Montoliu C, Valles S, Renau-Piqueras J, Guerri C (1994) Ethanol-induced oxygen radical formation and lipid peroxidation in rat brain: effect of chronic alcohol consumption. J Neurochem 63:1855–1862
Lindi C, Montorfano G, Marciani P (1998) Rat erythrocyte susceptibility to lipid peroxidation after chronic ethanol intake. Alcohol 16:311–316
Acknowledgements
This work was supported by the Ministry for Science, Technology and Environmental Protection of Serbia, Grant #145029B.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rašić-Marković, A., Krstić, D., Vujović, Z. et al. Modulations of rabbit erythrocyte ATPase activities induced by in vitro and in vivo exposure to ethanol. Mol Cell Biochem 308, 111–116 (2008). https://doi.org/10.1007/s11010-007-9618-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-007-9618-z