Abstract
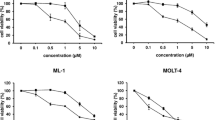
Human chronic myelogenous leukemia (CML) is a stem cell driven hematological malignancy which shows resistance to existing therapeutics. This property of CML accentuates the necessity to develop alternative anti-CML therapeutic agents. Herein, we have evaluated the anticancer activity of a novel anticancer peptide, Brevinin-2R and its two analogues, Brevinin-2R-C and Brevinin-2R-D regarding their inhibitory activity against K562 cells. Various cell-based analyses have been conducted to analyze the effects of these peptides and their mechanism of action. Hematotoxicity assay was performed to determine their hemolytic activities. MTT and neutral red uptake assays were conducted to examine anti-proliferative effects, propidium iodide (PI) staining to monitor the DNA content in different phases of cell cycle and Annexin V/PI staining to detect the apoptosis induction for the peptides. Our findings indicated that these peptides are capable of diminishing the cell growth and inducing apoptosis and cell cycle arrest. Brevinin-2R and its two analogues inhibited cell proliferation through strong cell cycle arrest in G2/M phase leading to apoptosis induction. The cytotoxicity of Brevinin-2R was higher than that of its two derivatives. These observations have provided new insights into the therapeutic activity of Brevinin-2R and its two analogues and suggest that these peptides have the potential to act as anticancer agents in treatment of K562. Further in vivo investigations on the therapeutic potential of Brevinin-2R and its two analogues are required to get a better grasp of their mechanism of action.




Similar content being viewed by others
References
Asoodeh A, Naderimanesh H, Mirshahi M, Ranjbar B (2004) Purification and characterization of an antibacterial, antifungal and non hemolytic peptide from Rana Ridibunda. J Sci Islam Repub Iran 15(4):303–309
Barras D, Widmann C (2011) Promises of apoptosis-inducing peptides in cancer therapeutics. Curr Pharm Biotechnol 12:1153–1165
Branford S et al (2014) Prognosis for patients with CML and > 10% BCR-ABL1 after 3 months of imatinib depends on the rate of BCR-ABL1 decline. Blood 124:511–518
Brown KL, Hancock RE (2006) Cationic host defense (antimicrobial) peptides. Curr Opin Immunol 18:24–30
Chan S-C, Hui L, Chen HM (1998) Enhancement of the cytolytic effect of anti-bacterial cecropin by the microvilli of cancer cells. Anticancer Res 18:4467–4474
Chu S, Holtz M, Gupta M, Bhatia R (2004) BCR/ABL kinase inhibition by imatinib mesylate enhances MAP kinase activity in chronic myelogenous leukemia CD34 + cells. Blood 103:3167–3174
Connor J, Bucana C, Fidler IJ, Schroit AJ (1989) Differentiation-dependent expression of phosphatidylserine in mammalian plasma membranes: quantitative assessment of outer-leaflet lipid by prothrombinase complex formation. Proc Natl Acad Sci 86:3184–3188
Cruciani RA, Barker JL, Zasloff M, Chen H-C, Colamonici O (1991) Antibiotic magainins exert cytolytic activity against transformed cell lines through channel formation. Proc Natl Acad Sci 88:3792–3796
Dennison SR, Harris F, Phoenix DA (2017) Maximin H5 is an anticancer peptide. Biochimie 137:29–34
Dumka D et al (2009) Activation of the p38 Map kinase pathway is essential for the antileukemic effects of dasatinib. Leuk Lymphoma 50:2017–2029
Evdokiou A, Bouralexis S, Atkins GJ, Chai F, Hay S, Clayer M, Findlay DM (2002) Chemotherapeutic agents sensitize osteogenic sarcoma cells, but not normal human bone cells, to Apo2L/TRAIL-induced apoptosis. Int J Cancer 99:491–504
Ghavami S et al (2008) Brevinin-2R1 semi-selectively kills cancer cells by a distinct mechanism, which involves the lysosomal-mitochondrial death pathway. J Cell Mol Med 12:1005–1022
Goldman JM, Melo JV (2001) Targeting the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med 344:1084–1086
Goldman JM, Melo JV (2003) Chronic myeloid leukemia—advances in biology and new approaches to treatment. N Engl J Med 349:1451–1464
Hegde M et al (2017) A benzothiazole derivative (5 g) induces DNA damage and potent G2/M arrest in cancer cells. Sci Rep 7:2533
Horita M et al (2000) Blockade of the Bcr-Abl kinase activity induces apoptosis of chronic myelogenous leukemia cells by suppressing signal transducer and activator of transcription 5–dependent expression of Bcl-xL. J Exp Med 191:977–984
Jamadi RH, Yaghoubi H, Sadeghizadeh M (2017) Brevinin-2R and derivatives as potential anticancer peptides: synthesis, purification, characterization and biological activities. Int J Pept Res Ther 25:151–160
Jenssen H, Hamill P, Hancock RE (2006) Peptide antimicrobial agents. Clin Microbiol Rev 19:491–511
Liu B et al (2017) Design of novel antimicrobial peptide dimer analogues with enhanced antimicrobial activity in vitro and in vivo by intermolecular triazole bridge strategy. Peptides 88:115–125
Mangoni ML, Fiocco D, Mignogna G, Barra D, Simmaco M (2003) Functional characterisation of the 1–18 fragment of esculentin-1b, an antimicrobial peptide from Rana esculenta. Peptides 24:1771–1777
Mehrnejad F, Naderi-Manesh H, Ranjbar B, Maroufi B, Asoodeh A, Doustdar F (2008) PCR-based gene synthesis, molecular cloning, high level expression, purification, and characterization of novel antimicrobial peptide, brevinin-2R, in Escherichia coli. Appl Biochem Biotechnol 149:109–118
Minn I, Kim HS, Kim SC (1998) Antimicrobial peptides derived from pepsinogens in the stomach of the bullfrog, Rana catesbeiana. Biochem Biophys Acta 1407:31–39
Momeny M et al (2017a) Anti-tumour activity of tivozanib, a pan-inhibitor of VEGF receptors, in therapy-resistant ovarian carcinoma cells. Sci Rep 7:45954
Momeny M et al (2017b) Dacomitinib, a pan-inhibitor of ErbB receptors, suppresses growth and invasive capacity of chemoresistant ovarian carcinoma cells. Sci Rep 7:4204
Papo N, Shai Y (2003) Can we predict biological activity of antimicrobial peptides from their interactions with model phospholipid membranes? Peptides 24:1693–1703
Papo N, Shai Y (2005) Host defense peptides as new weapons in cancer treatment. Cell Mol Life Sci CMLS 62:784–790
Papo N, Shahar M, Eisenbach L, Shai Y (2003) A novel lytic peptide composed of dl-amino acids selectively kills cancer cells in culture and in mice. J Biol Chem 278:21018–21023. https://doi.org/10.1074/jbc.M211204200
Pushpanathan M, Gunasekaran P, Rajendhran J (2013) Antimicrobial peptides: versatile biological properties. Int J Pept 2013:15
Risso A et al (2002) BMAP-28, an antibiotic peptide of innate immunity, induces cell death through opening of the mitochondrial permeability transition pore. Mol Cell Biol 22:1926–1935
Sah B, Vasiljevic T, Mckechnie S, Donkor O (2015) Identification of anticancer peptides from bovine milk proteins and their potential roles in management of cancer: a critical review. Compr Rev Food Sci Food Saf 14:123–138
Sotomayor S, Muñoz-Moreno L, Carmena MJ, Schally AV, Sánchez-Chapado M, Prieto JC, Bajo AM (2010) Regulation of HER expression and transactivation in human prostate cancer cells by a targeted cytotoxic bombesin analog (AN-215) and a bombesin antagonist (RC-3095). Int J Cancer 127:1813–1822
Stark M, Liu L-P, Deber CM (2002) Cationic hydrophobic peptides with antimicrobial activity. Antimicrob Agents Chemother 46:3585–3590
Suk F-M, Chang C-C, Lin R-J, Lin S-Y, Liu S-C, Jau C-F, Liang Y-C (2018) ZFP36L1 and ZFP36L2 inhibit cell proliferation in a cyclin D-dependent and p53-independent manner. Sci Rep 8:2742
Thennarasu S, Nagaraj R (1995) Design of 16-residue peptides possessing antimicrobial and hemolytic activities or only antimicrobial activity from an inactive peptide. Int J Pept Protein Res 46:480–486
Utsugi T, Schroit AJ, Connor J, Bucana CD, Fidler IJ (1991) Elevated expression of phosphatidylserine in the outer membrane leaflet of human tumor cells and recognition by activated human blood monocytes. Can Res 51:3062–3066
Wang X et al (2018) Autophagy enhanced antitumor effect in K562 and K562/ADM cells using realgar transforming solution. Biomed Pharmacother 98:252–264
Zwaal RF, Schroit AJ (1997) Pathophysiologic implications of membrane phospholipid asymmetry in blood cells. Blood 89:1121–1132
Acknowledgements
The authors wish to thank Islamic Azad University of Ardabil for supporting the conduct of this research. This study was supported by Grant No. 9775 from Shahroud University of Medical Sciences.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All author declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with animals performed by any of the authors. This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hassanvand Jamadi, R., Khalili, S., Mirzapour, T. et al. Anticancer Activity of Brevinin-2R Peptide and its Two Analogues Against Myelogenous Leukemia Cell Line as Natural Treatments: An In Vitro Study. Int J Pept Res Ther 26, 1013–1020 (2020). https://doi.org/10.1007/s10989-019-09903-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-019-09903-6