Abstract
The field of nanofluids has received interesting attention since the concept of dispersing nanoscaled particles into a fluid was first introduced in the later part of the twentieth century. This is evident from the increased number of studies related to nanofluids published annually. The increasing attention on nanofluids is primarily due to their enhanced thermophysical properties and their ability to be incorporated into a wide range of thermal applications ranging from enhancing the effectiveness of heat exchangers used in industries to solar energy harvesting for renewable energy production. Owing to the increasing number of studies relating to nanofluids, there is a need for a holistic review of the progress and steps taken in 2019 concerning their application in heat transfer devices. This review takes a retrospective look at the year 2019 by reviewing the progress made in the area of nanofluids preparation and the applications of nanofluids in various heat transfer devices such as solar collectors, heat exchangers, refrigeration systems, radiators, thermal storage systems and electronic cooling. This review aims to update readers on recent progress while also highlighting the challenges and future of nanofluids as the next-generation heat transfer fluids. Finally, a conclusion on the merits and demerits of nanofluids is presented along with recommendations for future studies that would mobilise the rapid commercialisation of nanofluids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Energy is a very important quantitative property that must be transferred before any system can perform work. The transfer of energy can be done by either work or heat [1]. Heat is transferred from one system to another when there exists a temperature difference between the two systems and travels from high to low temperatures [2]. The science that describes the means and rate in which thermal (heat) energy is transferred is known as heat transfer. Heat transfer applications are experienced in our daily life; the human body, for instance, is constantly emitting heat, and humans adjust their body temperature to suit environmental conditions using clothing. Heat transfer is also used in our buildings to regulate temperature [3] and is necessary for cooking, refrigeration and drying. It is also directly applied in car radiators [4] and for temperature control in electronic devices [5]. Heat transfer is used in solar thermal collectors to convert solar energy to heat and power [6, 7] and used in thermal control elements in spacecraft [8]. In many of these devices, heat needs to be dissipated at a rapid rate to ensure effective operation and maximum efficiency within the system [9]. As technology evolves, devices have become smaller and thus require better thermal management. Essentially, the more compact the size, the larger the requirement for effective cooling technology [10]. Therefore, heat transfer enhancement is a very important area in thermal engineering.
Several techniques have been considered to improve the heat transfer coefficient between the working fluids and the fluid contact surfaces [11, 12]. Conventional heat transfer fluids such as water, thermal oils and ethylene glycol/water have some limitations as their thermal properties are quite low when compared to those of solids, as shown in Fig. 1. The improvement in the thermal properties of these fluids through the addition of nanoscaled particles has led to an evolution in the study of heat transfer fluids. The suspension of these solid particles in the base fluid enhances the energy transmission in the fluid leading to improved thermal conductivity properties and better heat transfer characteristics [13]. The resultant fluids have been seen to possess higher values of thermal conductivity [14, 15]. Choi and Eastman [15] were the first to name such fluids as nanofluids. Nanofluids are the engineered colloidal suspension of nanoscaled particles (10–100 nm) in a base fluid [16]. These particles are generally metals, metallic oxides or other carbon-based elements. Over a century ago, Maxwell [17] was the first to discuss the suspension of micro-scaled particles into a fluid. However, microparticles settled rapidly in the fluid leading to abrasion and clogging in the flow channel, limiting further research into suspensions in fluids. Furthermore, these fluids did not exhibit the significant enhancement witnessed today with the use of nanofluids. The introduction of nanoparticles has allowed for further investigation into colloidal dispersion in fluids. Nanoparticles are more stable when dispersed in fluids and tend to improve on the thermal properties of the fluids. Some other properties of nanofluids which make them adequate heat transfer fluids include the Brownian motion of particles, particle/fluid nanolayers and their reduced pump power when compared to pure liquids to achieve intensified heat transfer.
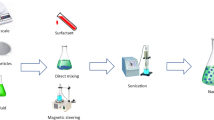
Despite these benefits, nanofluids still possess some application-based limitations. Issues of sedimentation and aggregation in the fluid have been raised, although the use of ultra-sonication, pH modulation, magnetic stirring and the addition of surfactants has been recorded to improve the stability of the nanofluids [18]. Also, increasing the fluid circulation rate in the device reduces the possibilities of sedimentation, although this can lead to an erosion of heat transfer in the device or flow stream. Particles of larger sizes also tend to clog the flow channel, and there have been cases of pressure loss recorded in some devices due to the marginal increase in viscosity. Nanofluids are also expensive to prepare and toxic due to the reactive nature of the nanoparticles [19]. Over the past decade, emphasis on nanofluids research has been more apparent as shown in Fig. 2, which illustrates the number of publications involving nanofluids since 2010. These studies include those related to their preparation, characterisation, measurement of their physical properties and their utilisation in various applications. The data presented in Fig. 2 were obtained by searching the word “nanofluids” in the Scopus database against titles, abstracts and keywords over the period presented. The search illustrates that approximately 3165 papers were published in 2019 alone, and this trend is expected to increase in the coming years. Incidentally, several review papers related to the heat transfer properties and application of nanofluids were published in 2019. The reviews include solar collector applications [20, 21], a review of nanofluids in heat exchangers [22, 23], review of nanofluids in heat pipes [24], radiator cooling [25], electronic cooling [5] and also a review on various thermophysical properties of nanofluids [26, 27]. A list of some of the review papers published in 2019 is listed in Table 1.
Owing to the increasing number of studies relating to nanofluids, there is a need for a holistic review of the progress and steps taken in 2019 concerning their application in heat transfer devices. This study adopts a retrospective look at the year 2019 by reviewing the progress made in the area of nanofluids preparation, nanofluid thermophysical properties measurements and the applications of nanofluids in various heat transfer devices including solar collectors, heat exchangers, refrigeration systems, radiators, thermal storage systems and electronic cooling. The study aims to update readers on recent progress in nanofluid synthesis and application. The study also seeks to highlight the challenges and prospects of nanofluids as the next-generation heat transfer material.
Preparation of nanofluids
The method used in the preparation of nanofluids is important in the study of the stability and thermophysical behaviour of nanofluids [57]. The preparation steps are also vital in estimating the degree to which the nanofluids are employed in heat transfer systems [58], [59]. In this section, the studies related to nanofluid stability and their synthesis techniques are discussed. Nanofluids are produced by suspending particles of nanosize dimensions in the traditional heat transfer fluids such as water, oils, acetone and glycols [60]. A wide range of nanoparticles have been utilised in the formation of nanofluids, some of these include:
-
(1)
Carbon nanoparticles (such as MWCNT, SWCNT, Gn, GO, graphite, diamond and fullerene).
-
(2)
Metal nanoparticles (such as Ag, Al, Au, Co, Cu and Fe).
-
(3)
Metal oxide nanoparticles (such as Al2O3, CeO2, CuO, Fe3O4, TiO2 and ZnO).
-
(4)
Others (such as Si, AlN-C, CoFe2O4, SiC, Field’s alloy nanoparticles, ZnBr2 and SiO2).
Nanofluids can be unstable due to the strong Van der Waals interactions and cohesive forces between nanoparticles. Therefore, the preparation technique used is extremely important in others to break down these forces and produce stable nanofluids. Different methods have been used to avoid nanoparticle agglomeration and improve the stability of nanofluids, such as pH control, surfactant addition, ultrasonic agitation, magnetic stirring, functionalisation and high-pressure homogenisation [114] experimentally measured the thermal conductivity of MWCNT–TiO2/ethylene glycol nanofluid and obtained that an increase in volume concentration of the nanofluid tends to increase the thermal conductivity of the hybrid nanofluid. The study considered volume concentration between 0.05 and 1% and observed that at a volume concentration of 1%, maximum enhancement (40.1%) in thermal conductivity was obtained. The study also developed an artificial neural network (ANN) model to predict the thermal conductivity values obtained from the experiment. Also, Alarifi et al. [115] developed an ANN model from their experimental results to predict the thermal conductivity of MWCNT–TiO2/thermal oil nanofluid.
As shown in Table 4, while many studies have begun to use the artificial neural network (ANN) models in thermal conductivity prediction, some others have proposed regression-based correlation equations to fit results obtained from their experiments. Moldoveanu et al. [116] conducted an experimental study on the thermal conductivity variation of Al2O3–TiO2/water nanofluid at volume concentration between 0.25 and 1% and proposed a correlation model to predict the thermal conductivity.
In terms of unique conventional nanofluids, Essajai et al. [117] studied the effect of particle shape on the thermal conductivity of nanofluids. The study was performed using a one-dimensional (1-D) network of interconnected gold nanoparticles (IAuNPs) and spherical Au nanoparticles. It was observed that IAuNPs in base fluids were more effective in improving the thermal conductivity of nanofluids than spherical Au particles suspended in a base fluid. Applying the one-step synthesis technique, the stability and the thermal conductivity measurement of MWCNTs/Jatropha seed oil nanofluid were investigated [118]; this environment-friendly nanofluid showed a thermal conductivity enchantment of 6.76% at a mass concentration of 0.8%.
ANN has also been used to predict the effect of particle aggregation on the thermal conductivity of nanofluids [119]. Mirsaeidi and Yousef [120] used ANN to predict the thermal conductivity, density and viscosity of carbon quantum dots nanofluids using water, ethylene glycol and EG–water (60:40) as base fluids. Motlagh et al. [121] used gene expression programming to propose a correlation that estimates the thermal conductivity of Al2O3 and CuO–water-based nanofluid based on experimental data from the literature. Going forward, it is expected that there will be an increase in research and development exploring the possible advantages of using ternary hybrid nanofluids. In this regard, the study conducted by Mousavi et al. [122] has already demonstrated that the thermal conductivity of CuO–MgO–TiO2/water nanofluid is enhanced by 78.6% at a mass fraction of 0.1. For the reader’s convenience, the authors have summarised the work on thermal conductivity studies for hybrid nanofluids in the year 2019 in Table 4.
Viscosity of nanofluids
The viscosity of a fluid is important in understanding both the heat transfer and the flow behaviour of the fluid. Several experimental studies have been carried out to understand the behaviour of nanofluids. The available research on the topic is not limited to experiments alone as molecular dynamics simulations have also been used to explain the viscosity of nanofluids [136]. Dehghani et al. [137] analysed the effect of temperature and mass fraction of Al2O3 and WO3 nanoparticles in water and liquid paraffin. Their findings showed that the viscosity of both nanofluids is increased only by adding a certain number of nanoparticles to both fluids. Regarding the shear rates, the viscosity of water-based nanofluids is constant, which indicates a Newtonian behaviour, while that of paraffin does not remain constant at different shear rates, and at a low amount of shear rate the viscosity achieves higher value, indicating a non-Newtonian behaviour for liquid paraffin-based nanofluids. Finally, they presented a correlation based on temperature, nanoparticle concentration and the physical properties of both the nanoparticle and base fluid for predicting the viscosity of aqueous and non-aqueous nanofluids. Ye et al. [138] extensively covered the viscosity of nanofluids that predate 2019. In 2019, as with thermal conductivity studies, there has been a trend towards hybrid nanofluids. The significance of viscosity in lubrication applications has been seen in many investigations related to oil-based nanofluids. Using the ultrasonic-assisted process, Barai et al. [85] synthesised graphene oxide–Fe3O4/water nanofluid at volume concentrations between 0.01 and 0.2%. The study obtained a maximum viscosity enhancement of 41%. Studying Fe–CuO/EG–water nanofluid, Bahrami et al. [139] obtained that the backward propagation methods presented the least error in predicting dynamic viscosity. Bahrami et al. [139] deduced that when the hybrid nanofluids volume concentration is below 0.1%, the Fe–CuO/EG–water nanofluid exhibited Newtonian behaviour. However, when Fe–CuO/EG–water nanofluid volume concentration is above 0.25% the behaviour of the fluid changed.
As shown in Table 5, while many studies have proposed various correlation models to predict the viscosity behaviour of nanofluids, the more accurate models proposed are the artificial neural network (ANN) models. Ruhani et al. [140] investigated the effects of volume concentration and fluid temperature on the viscosity of hybrid nanofluids. The correlation model proposed in this study demonstrated a 1.8% margin of deviation between experimental values and correlation results. Viscosity enhancement was about 80% when the volume fraction was 2%.
Other types of conventional nanofluids were also studied; Mousavi et al. [141] conducted an experimental investigation into the viscosity measurements of MoS2/diesel oil nanofluid at particle concentration between 0.1 and 0.7%. The study observed that the viscosity increased by 7.04% when volume concentration was 0.7%. Hameed et al. [142] synthesised an eco-friendly MWCNTs-Kapok seed oil nanofluid using a one-step method, at a constant nanoparticle concentration of 0.1%.
Considering all of the experimental viscosity measurements conducted, the relationship between viscosity and both temperature and particle concentration is apparent. Naturally, the viscosity of nanofluids increases with an increase in particle concentration, and this is observed in virtually all measured experiments. The viscosity of nanofluids decreases with an increase in temperature, and this is also observed in all measured experiments; this behaviour is expected as entropy is increased as particles gain thermal energy. However, the relationship between particle concentration and rheology is not as apparent. Considering the sample size alone as illustrated in Table 5, it can be observed that there exists no clear pattern between rheological behaviour and particle concentration in nanofluids. Rheological behaviour appears to vary from material to material.
Specific heat of nanofluids
The specific heat capacity of fluids is important in understanding both the heat transfer and the energy content of thermal systems. While significant research has focused on both viscosity and thermal conductivity, studies relating to the specific heat capacity of nanofluids are not as advanced. However, the specific heat capacity of fluid bears significance in thermal storage applications. Therefore, many studies regarding specific heat capacity often use molten salt as their base nanofluid. Moldoveanu and Minea [153] experimentally measured the specific heat of both Al2O3–TiO2/water nanofluids and Al2O3–SiO2/water nanofluids at volume concentration between 1 and 3.0%. A correlation model was determined from the measured specific heat capacity values. It is important to note that the correlation model had an average deviation of 11% when compared to experimental specific heat values. However, when the mixture theory model was used to predict the nanofluids’ specific heat capacity values, the deviation was as high as 19%.
The effect of particle size and volume fraction on the specific heat of SiO2 molten salt nanofluid was investigated by Li et al. [154]. Using SiO2 nanoparticle with sizes of 10, 20, 30 and 60 nm, SiO2 molten salt nanofluid was synthesised at particle concentration between 0.5 and 2%. Addition of particles to molten salt increases the specific heat capacity for all of the volume concentrations and particle sizes considered. An important point to note is that the particle concentration and particle size with the most specific heat enhancements were 1% and 20 nm, respectively.
Using SiO2, Al2O3 and TiO2 nanoparticles, three conventional nanofluids were synthesised by Hassan and Banerjee [155]. The study aimed to predict the specific heat capacity of metal oxide molten nitrate salt nanofluids using a multilayer perceptron neural network (MLP-ANN). The ANN model proposed was more accurate when compared to classical prediction methods [155]. Alade et al. [156] also considered a machine learning approach by applying a support vector regression model optimised with a Bayesian algorithm to predict the specific heat capacity of Al2O3 ethylene glycol nanofluids. The proposed model also exhibited a high degree of accuracy with the root-mean-square error (RMSE) equivalent to 0.0047.
From Table 6, while the specific heat of molten salts increases with the addition nanoparticles, in experiments involving MWCNTs PEG 400 nanofluid, Al2O3–water nanofluid, Fe–water nanofluid and Al2O3–Fe nanofluid the specific heat of the base fluid exceeds that of the nanofluids.
Factors affecting nanofluids stability and thermophysical properties
The main factors affecting the thermophysical properties of nanofluids includes the morphology and concentration of nanoparticles, aggregation in the nanofluids and the sonication time used in its preparation [158]. The stability of nanoparticles suspended in a fluid is a very important parameter that affects both the rheological and thermophysical behaviours of the resultant nanofluids. Brownian motion causes the particles to collide with one another leading to cluster formation in the base fluid. These cluster formations or aggregation are controlled by a variety of internal forces between the base fluid and the nanoparticles such as the Van der Waals forces of attraction between the particles [159]. The aggregates begin to crystallise as their density exceeds that of the base fluid and affects the stability of the nanofluids over time [152]. Some of the factors that affect the stability of the nanofluids include the method of preparation of the nanofluids [66], agitation and sonication time [160,161,162], pH of the nanofluids [152], the addition of surfactants [163, 164] and surface charge density of the nanoparticles [158]. Asadi et al. [165] reviewed the effect of sonication on the stability and thermophysical properties of nanofluids. The study concluded that while there exists an optimum sonication time where thermal conductivity is maximum, and viscosity is least, more research is required to determine this optimum value, as it appears to differ for different nanofluids. Khan and Arasu [166] also reviewed the effects of nanoparticle synthesis techniques on the stability and thermophysical behaviour of nanofluids. The study importantly noted that there appears to be no standard method for stability measurements; this makes it difficult to compare stability across different papers. This is a problem because of the significant differences in reported fluid stability; this can range from days in some studies to months in others.
The values of the thermophysical properties of nanofluids are sensitive to the volume and size of nanoparticles used, the temperature of the mixture and the use of surfactants [167]. Yang et al. [168] investigated the thermal conductivity of graphene oxide/water nanofluids with a mass concentration range of 0–1.5%. Their result showed that as the mass fraction of nanoparticles increased, the thermal conductivity enhancement increased. Also, at a pH of 8, the nanofluids showed maximum stability with a maximum thermal conductivity enhancement of 48.1%. This indicated that the pH was a significant parameter in both its stability and thermal conductivity. The authors attributed the thermal enhancement observed to the increased Brownian motion of particles and molecules of the base fluid as temperature increased. Yang et al. [169] also studied the thermal conductivity behaviour of zinc nanopowder in SAE 50 engine oil and recorded an increase in the thermal conductivity of the nanolubricant as the volume concentration of nanoparticles was increased. They recorded a maximum thermal conductivity enhancement of 8.74% and attributed this to the effects of increased Brownian motion of particles in the lubricant as temperature raises. The thermophoresis effect was another factor they highlighted that affected the thermal conductivity enhancement.
Rostami et al. [71] examined the thermal conductivity of GO–CuO water/EG (50:50) hybrid nanofluid at a temperature of 25–50 °C and particle volume concentration of 0.1–1.6%. Their investigation observes a 46% enhancement in thermal conductivity, which is higher than the enhancement of using single nanomaterial. Mahyari et al. [73] investigated the thermal conductivity GO/SiC (50:50)/water hybrid nanofluid at volume concentrations between 0.05 and 1%. Their investigation reveals that the effect of the volume concentration of nanoparticles was more significant than the effect of increasing temperature. Importantly, the studies observed that the enhancement in thermal conductivity of their hybrid nanofluid was more than the reported thermal conductivity enhancement using GO or SiC individually. Hybrid nanofluids not only affect the thermal conductivity, but also enhance the stability of nanofluids.
Heat transfer mechanisms of nanofluids
The main benefit of using nanofluids is their enhanced thermal transport which results in improvements in the thermal conductivity of traditional heat transfer fluids. As previously outlined, several parameters influence the thermal conductivity enhancement and include nanoparticle type, nanoparticles size, nanoparticles concentration, temperature, type of base fluid and the thermophysical properties of both the base fluid and the nanoparticles. Over the last three decades, since the introduction of nanofluids in 1995, the explanations behind the enhanced heat transfer of nanofluids have been attributed to several mechanisms. The size and the large number of particles interacting with the base fluid present a challenge to properly understanding the nanoscale effects that support the improved thermal properties observed in the literature. Mahian et al. [108, 170] studied the mechanisms that would aid the simulation of nanofluids flow. They highlighted that forces such as drag, lift, Brownian motion, thermophoresis, Van der Waals and electrostatic double-layer forces had a significant effect on the thermal and rheological behaviours of nanofluids.
Brownian motion is defined as the uncontrollable random motion of particles within the fluid due to the collision between slow moving and higher velocity particles. Brownian motion occurs as a result of thermal diffusion, and this phenomenon is increased at higher temperatures, low viscosity and smaller particle size. As promoted by the scientific community, the random collision of particles within the fluid remains the primary reason for the thermal conductivity enhancement observed with nanofluids [73, 79, 92]. However, Jang and Choi [171] provided three types of collisions that occur due to the rising temperature of nanofluids: collisions between the molecules of the base fluid, collisions between base fluid molecules and the nanoparticles, and the collisions between nanoparticles due to Brownian motion. They concluded that the effect of Brownian motion on thermal conductivity enhancement had the least effect among the three types of collisions.
Keblinski et al. [172] was the first to introduce the idea of nanolayers and their effect in nanofluid thermophysical behaviour. The nanolayer is known as the solid-like structure or the interfacial layer between the solid surface and the first layer of the fluid in contact with the solid surface. A structured, layered arrangement of the fluid molecules around the surface of the nanoparticles was observed. These layers behaved like solids and act as a thermal bridge for the heat transfer process enhancing the overall thermal conductivity of the fluid. In the solid–solid interface, this layer acts as a barrier of heat transfer due to incomplete contact between solid surfaces. However, it is not the case for the solid–liquid interface as the aligned interfacial shell in the nanoparticle suspension would make heat transfer across the interface effective. Yu and Choi [173] presented a modified Maxwell model to account for the effect of nanolayers on the thermal conductivity of nanofluids. Their results proved that the thermal model is enhanced as a result of accounting for this factor. **ing [317] all show an increase in the dimensionless heat transfer parameter with the addition of nanoparticles. These studies prove the tremendous potentials of nanofluids in the electronics and data storage industries. Also, the heat transfer behaviour of nanofluids in magnetic fields has shown promising potentials [318].
Vishnuprasad et al. [319] experimentally evaluated the cooling performance of microwave-assisted acid-functionalised graphene (MAAFG) in water. The characterisation of the nanofluid showed that the MAAFG nanofluid had a 55.38% enhancement in thermal conductivity. The effect of varying the flow rate and nanoparticle volume concentration on the heat transfer coefficient and processor temperature was studied, and the results show that at 0.2 Vol%, there was an increase in the convective heat transfer coefficient by 78.5%. The processor temperature was also decreased by 15%, although a 5% pressure drop was recorded at 0.2 Vol% and a flow rate of 10 mL s−1. Joy et al. [320] investigated the use of Cu–water and Al–water to increase the critical heat flux (CHF) limit in a heat pipe for electronic cooling. The result of the study demonstrated that nanofluids increased the CHF by 140% at a mass concentration of 0.01%. Both nanoparticle concentrations represented the optimum value of CHF for both nanofluids without preheating. Zing and Mahjoob [321] theoretically investigated the use of single- and multijet im**ements through a porous channel for electronics cooling applications. The study evaluated the effect of two different coolants in their system: water and TiO2–water nanofluids at a volume concentration of 5%. Results demonstrate that the use of TiO2 nanofluid decreased the base temperature of the device more effectively than using water. For enhanced heat transfer in electronic cooling, Bezaatpour and Goharkhah [322] designed a mini heat sink with porous fins operating with a magnetite nanofluid of Fe3O4–water at volume concentrations up to 3%. The study recorded an increase in heat transfer of 32% with the use of the ferrofluids at 3 Vol% and Re of 1040. The pressure drop also recorded a decrease of 33% with the use of the ferrofluids.
Al-Rashed et al. [323] evaluated the first and second law performance of a non-Newtonian nanofluid of CuO and 0.5% carboxymethyl cellulose (CMC) in water for use in a microchannel heat sink (MCHS). Figure 16 illustrates an offset strip-fin MCHS with a description of its geometric parameters and imposed boundary conditions. By varying the nanoparticle concentration and Reynolds number, the effect of the nanofluids on the surface temperature of the CPU was observed. The results demonstrate that increasing Reynolds number adversely affected the frictional entropy generation and pressure drop. The nanofluid also reduced the surface temperature of the CPU and entropy generation rate in the system. A 2.7% decrease in the entropy generation rate of the CPU was attained at 1 Vol% and Re of 300. At 1 Vol% and Re of 700, the CMC/CuO water had an optimal ratio of heat transfer to the pressure drop of 2.29. Qui et al. [324] investigated the interfacial transport between vertically aligned carbon nanotube and electronic heat sinks. Their results show that CNT reduced the thermal contact resistances from 10 mm2K/W to 0.3 mm2K/W. Other studies related to the use of nanofluid in improving heat transfer in electronic devices are detailed in Table 9.
Nanofluids in automobile radiators
The thermal management of automobile engines is necessary for the effective and efficient operation of the automobile. Figure 17 illustrates a schematic diagram of a car radiator which functions as a heat exchanger that disperses the heat generated from the operation of the engines. Recently, the use of nanofluids as alternative coolants in radiators have been investigated. Elsaid [341] experimentally investigated the performance of an engine radiator using nanofluids in the hot arid climate of Cairo, Egypt. Two nanoparticles Al2O3 and Co3O4 are used in varying concentrations in a base fluid of EG/water at 0:100%, 10:90% and 20:80%. A schematic of his experimental set-up for investigating nanofluids effectiveness in radiators is illustrated in Fig. 18. The study confirms that the use of Co3O4/EG–water results in a more favourable thermal performance than that of Al2O3/EG–water. The cobalt oxide also contributed to larger energy savings when compared to alumina. The nanoparticles enhanced the Nusselt number by 31.8%; however, this was at the expense of an increase of 16% in friction factor. This reduction in friction factor resulted in the need for additional pump power for the nanofluids. It is essential to note that pump power was also intensified with the use of EG–water as the base fluid. The performance of a hybrid of Al2O3 nanocellulose dispersed in EG/water in a radiator was investigated by Naiman et al. [342], who recorded a maximum thermal conductivity at 0.9 Vol% and concluded that the nanofluids were more efficient than the use of EG–water. Al Rafi et al. [343] studied the heat transfer potential of Al2O3/EG–water and CuO/EG–water in a car radiator, revealing that the addition of EG into the water decreased the overall heat conductance by 20–25%. Moreover, experimental results demonstrate that Al2O3/EG–water at 0.1 Vol% and CuO/EG–water at 0.2 Vol% improved the heat transfer potential of the radiator by 30–35% and 40–45%, respectively.
Schematic diagram of the experimentation system used by Elsaid [341]
Kumar and Sahoo [344] analysed the energy and exergy performance of a wavy fin radiator using Al2O3–water nanofluid as a coolant. The effect of various nanoparticle shapes (spherical, brick and platelet) on the radiator’s effectiveness, pump power and heat transfer was also investigated; results show that the shape of the nanoparticles affected their performance in the radiator. Furthermore, it was observed that the spherical nanofluids had a 21.98% enhancement in heat transfer when compared to the platelet nanofluid. A 13% enhancement in the exergy efficiency of the spherical nanofluids determined that the use of spherical nanofluids performed better in comparison with nanofluids of other shapes. Contreras et al. [345] experimentally investigated the thermo-hydraulic performance of silver/EG–water and graphene/EG–water for use in a radiator. The study showed that silver/EG–water had an improved heat transfer rate of 4.7% when compared to EG–water, while the heat transfer using graphene nanofluid decreased by 11% and 3% at concentrations for 0.01 Vol% and 0.05 Vol%, respectively, when compared to water. The thermo-hydraulic performance coefficient of all nanofluids showed that nanographene at 0.1 Vol% and silver nanofluids at 0.05 Vol% had values of 1.5% and 2.5%, respectively, while graphene nanofluids at concentrations of 0.01 Vol% and 0.05 Vol% were not suitable for use in the radiator as they performed below EG–water. Other studies on the use of nanofluid in improving the performance of automobile radiators are detailed in Table 10.
Nanofluids in thermal storage
Thermal energy storage (TES) is a very important part of the utilisation, conservation and development of new and existing energy sources. The three forms of TES are chemical energy storage, sensible heat storage and latent heat storage. The difference between sensible and latent heat storage types is related to the phase transition of the thermal material used for storage. There is a phase transition before energy is released or stored in the Latent TES, while sensible TES does not require a phase change and operates mainly with the changing temperature of the material. Phase change materials (PCMs) can be used in both cases and is essential to the operation of the latent TES unit. The drawbacks of PCMs are their low thermal properties.
A classification of the various materials used in thermal energy storage is presented in Fig. 19. Highlighting the studies that investigate the effects of nanoparticles on the thermal performance of PCM, Bondareva et al. [357] investigated the heat transfer performance of the nano-enhanced phase change material system under the inclination influence. Studying the performance of paraffin enhanced with Al2O3 nanoparticles, they discovered that; for small inclinations of the cavity, when convective heat transfer dominates, an increase in the nanoparticles volume fraction leads to an increase in the melting time. Navarrete et al. [358] proposed the use of molten salt-based nanofluid for both sensible and latent energy storage. The molten salt nitrate would serve as the base fluid for the nano-encapsulated phase change materials (nePCM) consisting of Al-Cu alloy nuclei. Oxidation that occurs as a result of the metals been exposed to air would serve as an encapsulation over the nanoparticles. The study tested the resistance of the oxide shell to temperatures up to 570 °C, demonstrating that although the specific heat and by extension the sensible heat storage decreased with the presence of solid content, the phase change enthalpy and latent storage capacity increased by 17.8% at constant volume bases. Furthermore, the thermal conductivity of the salt nitrates increased with the addition of nanoparticles enhancing the heat transfer performance of the PCM nanofluid. Martin et al. [359] developed a novel nePCM from two fatty acids of capric acid (CA) and capric–myristic (CA-MA) using nSiO2 for thermal energy management in a building. The addition of the 1.5% nSiO2 significantly improved both the thermal conductivity and specific heat of nePCM. The thermal stability test after 2000 thermal cycles indicated that the addition of nanoparticles did not affect the thermal stability of CA, but slightly improved that of CA-MA. The sensible heat storage capacity of both fatty acids improved due to a 20% improvement in specific heat capacity at a volume concentration of 1%; however, the latent energy storage capacity of both fatty acids was lowered. The use of the nSiO2 nanoparticles strengthens on the initial weaknesses of the fatty acids as heat storage fluids as Fig. 20 illustrates.
Classification of the various thermal energy storage materials (modified from [362])
Organic PCMs that plot latent heat of fusion vs thermal conductivity [359]
Ding et al. [360] studied the use of two crystal forms of TiO2 nanoparticles (anatase referred to as A and rutile referred to as R) dispersed in water operating in a microchannel inside a PCM used to enhance the thermal storage in miniatured devices. The two nanofluids R-TiO2–water and A-TiO2–water were thermally tested, and both nanofluids confirmed to be stable. R-TiO2–water was more stable than A-TiO2, and the thermal conductivity of R-TiO2 was found to be higher than that of A-TiO2. The addition of TiO2–water in the microchannel at a volume concentration of 0.5%, 0.7% and 1.0% decreased the complete melting time of paraffin by 7.78%, 16.51% and 32.90% while increasing the complete solidification time by 7.42%, 15.65% and 22.57% in the solidification process. The use of nanofluids increased the melting and solidification pressure by less than 8% in both cases. Harikrishnan et al. [361] investigated the effect of Ni–ZnO nanocomposite dispersed in oleic acid on the thermal conductivity and phase change properties of the resulting nePCM. The thermal reliability along with the freezing and melting characteristics of the nePCM was studied, and the thermal conductivity of the nanofluids was confirmed to be higher than that of oleic acid. For the mass fraction considered, 0.3, 0.6, 0.9 and 1.2% of Ni–ZnO, the complete melting and solidification processes were enhanced by 7.03%, 14.06% 24.21%, 29.69% and 7.58%, 13%, 19.13%, 28.52%, respectively. The trend confirms that the time required in melting and freezing was lowered with the use of the nano-PCMs. Other studies related to the use of nanoparticles in thermal storage units are detailed in Table 11.
Nanofluids in refrigeration
Nanofluids can also be used in air conditioning and refrigeration systems. The negative environmental effect of using chlorofluorocarbons along with hydrofluorocarbons has propelled research into alternative refrigerants. Traditionally, vapour compression refrigeration systems (VCRSs) are used in the cooling industry; however, the major drawback to this system is the large compressor power requirement. An alternative heat-powered absorption refrigeration system (VARS) has been developed, although the coefficient of performance (COP) of these systems is still below those of the VCRS. Nanoparticles have been used to create new refrigerants known as nanorefrigerants which can improve the COP of both the VARS and VCRS and decrease the compression work of the VCRS.
Rahman et al. [376] analysed the effect of using nanoparticles in a refrigerant. The effect of the nanorefrigerant on the compression work and COP of the air conditioning system is observed. They observed that the addition of 5% SWCNT to R407c refrigerant at temperatures between 283 K and 308 K resulted in a reduction in the energy consumption of the compressor by 4%. Moreover, the nanorefrigerant had improved the thermal conductivity and specific heat values by 17.02% and 10.06%, respectively. The nanorefrigerant also enhanced the COP by 4.59% and reduced the compressor work by 34% when compared to conventional vapour compression refrigeration systems.
Jiang et al. [377] investigated the effect of 0.5% TiO2 and 0.02% SDBS on the COP of ammonia absorption refrigeration system (AARS). The experimental set-up of the test rig used in their investigations is illustrated in Fig. 21. Outcomes of the experiment were compared to that of 0.1%, 0.3% and 0.5% of TiO2 dispersed in ammonia water as a refrigerant. The results demonstrate that the addition of TiO2 to any of the concentrations studied significantly improved the COP of the AARS. It was observed that the further addition of 0.02% of SDBS improved the stability of the mixture and enhanced the COP by 27% as shown in Fig. 22. In conclusion, the improvement in COP of the AARS was strongly dependent not only on nanoparticle concentration but also on the number of nanoparticles stably dispersed in the base fluid. Jeyakumar et al. [378] investigated the use of three nanoparticles CuO, ZnO and Al2O3 in the refrigerant of a vapour compression system. The nanoparticles were added to refrigerant R134 at concentrations of 0.06%, 0.08% and 0.1% with 0.1% polyester oil as a lubricant. The results demonstrate an improvement in COP of 12.2% and 3.42% using the nanorefrigerant of CuO and Al2O3, respectively. Also, a reduction in the power consumption of 1.39% and 0.6% with CuO and Al2O3, respectively, was observed. Other studies related to the use of nanoparticles in compression and absorption refrigeration systems are given in Table 12.
Test rig for investigating the influence of TiO2 nanoparticles on AARS [377]
The COP of AARS with different mass fractions of TiO2 [377]
The use of nanofluids in many other devices has also been studied, and some of these include the application of nanofluids in solar still [389, 390] and also in mineral oil to enhance the insulating properties of high-voltage AC and DC transformers as proposed by Rafiq et al. [391].
Challenges and future prospects
Due to stability concerns with nanofluids, exponential improvements are required for nanofluids to reach their full potential as heat transfer fluids. The problems with stability are more obvious in liquids with low viscosity than liquids with high viscosity. Most of the current methods used to increase fluid stability appear to fall short in certain regards. pH modulation has demonstrated promising signs of improving the stability of nanofluids; however, acidic and basic solutions exponentially increase corrosion in metals and would thus render heat transfer system untenable. The addition of surfactants has the potential to improve nanofluids stability, however, at high-temperature surfactants tend to foam and decrease the overall efficiency of the system. The most promising technique for increasing fluid stability is by improving the synthesis techniques used. Incidentally, the most common method for synthesising nanofluid is the worst performing method for ensuring fluid stability. Green synthesis techniques demonstrate sufficient promise in improving stability; however, the thermal performance of the green-synthesised nanofluids is not normally as high as nanofluids synthesised by the two-step technique. Furthermore, there appears no standard for reporting the stability of nanofluids. Therefore, a generic standard for measuring nanofluid stability must be developed so that easy comparisons can be made across nanofluid types.
Another significant challenge is the theoretical unpredictability of the thermophysical behaviour of nanofluids. While many studies settle for regression-based correlation models to predict thermophysical properties, intelligent computing has also been widely used in the predictions. It is the opinion of the authors that because of the almost infinite variables that affect the thermophysical behaviour of nanofluids, intelligent computing would be the most accurate predicting the thermophysical behaviour of nanofluids. Therefore, a generic standard must be developed for labelling data obtained from the experiments measuring thermophysical properties of nanofluids. Develo** a global data bank will drastically improve the prediction accuracy of artificial neural network and machine learning models, saving unlimited research costs in conducting thermophysical behaviour measurements.
To improve numerical analysis models, further nanofluid heat transfer correlation studies are required for determining the Nusselt number correction equation. Many studies adopt the Nusselt number correlation equation proposed by Pak and Cho [392]; however, this model was developed for water, Al2O3–water and TiO2–water nanofluids and may not be particularly accurate for other nanofluids. More experiments using other nanofluids, especially for hybrid nanofluids, will further enlighten the field and improve the accuracy of numerical studies.
Finally, the classification of nanofluids must be improved. As nanofluid research increases, several unique types of fluids are synthesised. Previously, conventional fluid often implies fluids with a single-particle material, while hybrid nanofluid refers to a fluid with more than one nanoparticle material. However, it appears that further classifications are required as nanofluid have the potential to have an nth number of significant nanomaterials types present in the fluid. Some authors have sought to classify nanofluids with two significant nanomaterials type as “binary hybrid nanofluid” and nanofluids with three significant nanomaterials type as “ternary hybrid nanofluid”. It may be beneficial if classifications are conducted along these lines.
Conclusions and recommendations
The use of nanofluids as coolants in heat transfer devices has gained attention over the years. This study presents a detailed review of studies relating to the preparation, thermophysical property measurements and application of nanofluids in a range of thermal devices requiring efficient heat transfer published in 2019. Some of the areas reviewed include thermophysical models used in determining the properties of the nanofluids, mechanisms that support the enhanced thermal behaviours of nanofluids, and the application of different nanofluids in devices such as solar collectors, heat exchangers, electronics cooling and thermal storage. Based on the articles reviewed in this study, the following recommendations are made:
On the preparation of nanofluids;
-
Few studies on the preparation of nanofluids based on the one-step method are available, and this method has been proven to have better stability than the two-step method. More studies on the production of nanofluids using the one-step method are needed, as this could help in the development of more cost-effective means for the large-scale production of nanofluids.
Regarding the thermophysical properties of nanofluids:
-
An increase in the nanoparticle volume concentration leads to a decrease in the specific heat capacity of nanofluids in cases where the heat capacity of base fluids is higher than those of nanoparticles. Since a higher heat capacity is needed in coolants, further studies are required to assess how this phenomenon can be improved.
-
Many studies on the thermal behaviour of nanofluids were conducted for temperatures between 10 and 100 °C. The interaction mechanism of nanoparticles in base fluids for heat transfer at higher temperatures (greater than 100 °C) and cryogenic conditions requires further investigation.
-
There exist huge differences between the heat transfer predicted by the single-phase homogenous model and those obtained from experiments. More studies related to the development of other models (two-phase models) are required which allude to defining the conditions where the single-phase models can be applied to provide more accurate results.
-
There has been an increase in both the number and methods for develo** correlation models that predict the thermophysical properties of nanofluids. However, more correlation equations that predict the heat transfer (Nusselt number) and friction factor behaviours of many nanofluids are needed.
On studying the mechanisms that influenced the properties of nanofluids:
-
Knowledge of the dominant forces responsible for the behaviour of nanorefrigerants in various flow configurations requires further development.
-
An understanding of the impact of nanoparticle morphology (size and shape), nanoparticle mixture ratio (for hybrid nanofluids) on heat transfer augmentation is limited. More studies are needed to understand the impact of these on the performance of nanofluids in heat transfer devices.
Investigation on the various heat transfer devices:
-
Further studies are required, as there are contrasting reports on the effect of nanoparticle loading on the pressure drop and additional pump power requirement. While some studies claim that the effect of particle loading increases the pressure drop and consequently the pump power requirement of the system, others argue that when the heat transfer rate obtained using nanofluids is compared with that of conventional fluids, the nanofluids lowers the pump power requirements.
-
In heat exchangers and car radiators, the constant rate of heat transfer from the use of nanofluids leads to a reduction in the heat transfer surface. This can result in an improvement in the size and volume of these devices. Such improvements would lead to a reduction in the drag forces witnessed in vehicles and increase the performance of the engine.
-
The most common model used in the literature for the simulation of nanofluids remains the finite volume method. Further studies using other methods are needed for the comparison of the different numerical approaches.
-
Further studies on the effects of erosion of heat transfer and corrosion of flow channels resulting from the use of nanofluids, especially in high temperatures, are required. Both the short- and long-term impacts of sedimentation and nanoparticle deposition on the efficiency of heat transfer devices require investigation.
-
Few studies are available on the production cost and environmental impact of nanofluids. Such factors present huge hurdles to the commercialisation of nanofluids.
-
Further information on the effect of oxidisation of metallic nanoparticles used with phase change materials on the thermal performance of the thermal storage unit is required, especially during the melting phase.
Abbreviations
- AARS:
-
Ammonia absorption refrigeration system
- AFM:
-
Atomic force microscopy
- AG:
-
Arabic gum
- ANN:
-
Artificial neural network
- CA:
-
Citric acid
- CFD:
-
Computational fluid dynamics
- CHF:
-
Critical heat flux
- CMC:
-
Carboxymethyl cellulose
- CNT:
-
Carbon nanotubes
- COP:
-
Coefficient of performance
- CPC:
-
Compound parabolic collectors
- CPU:
-
Central processing unit
- CTAB:
-
Cetrimonium bromide
- DAPTC:
-
Direct absorption parabolic trough collector
- DASC:
-
Direct absorption solar collector
- DI:
-
Deionised
- DLS:
-
Dynamic light scattering
- EBT:
-
Eriochrome Black T
- EDX:
-
Energy-dispersive X-ray spectroscopy
- ETSC:
-
Evacuated tube solar collector
- FESEM:
-
Field emission scanning electron microscope
- FPC:
-
Flat plate collector
- FTIR:
-
Fourier-transform infrared spectroscopy
- GNP:
-
Graphene nanoplatelets
- HPSC:
-
Heat pipe solar collector
- HPSWH:
-
Heat pipe solar water heater
- HX:
-
Heat exchangers
- LFR:
-
Linear Fresnel reflectors
- MAAFG:
-
Microwave-assisted acid-functionalised graphene
- MCHS:
-
Microchannel heat sink
- MLG:
-
Multilayer graphene
- MWCNT:
-
Multiwall carbon nanotubes
- nePCM:
-
Nano-encapsulated phase change materials
- OA:
-
Oleylamine
- PAO:
-
Polyalphaolefin
- PCM:
-
Phase change materials
- PEG:
-
Polyethylene glycol
- PTSC:
-
Parabolic trough solar collectors
- PV:
-
Photovoltaic
- PVA:
-
Polyvinyl alcohol
- PVP:
-
Polyvinylpyrrolidone
- PVT:
-
Photovoltaic thermal collectors
- SDBS:
-
Sodium dodecylbenzene sulphonate
- SDS:
-
Sodium dodecyl sulphate
- SEM:
-
Scanning electron microscope
- SWCNT:
-
Single-wall carbon nanotube
- TEM:
-
Transmission electron microscope
- TES:
-
Thermal energy storage
- VARS:
-
Vapour absorption refrigeration system
- VCRS:
-
Vapour compression refrigeration systems
- Vol%:
-
Volume per cent
- XRD:
-
X-ray powder diffraction
- ZVI:
-
Zero-valent iron
References
Incropera FP, Bergman TL, Lavine AS, DeWitt DP. Fundamentals of heat and mass transfer. New York: Springer; 2011.
Cengel YA, Boles MA. Thermodynamics: an engineering approach. 8th ed. New York: McGraw-Hill; 2015.
Cengel YA. Heat transfer: a practical approach. 2nd ed. New York: McGraw-Hill; 2002.
Zhao N, Li S, Yang J. A review on nanofluids: data-driven modeling of thermalphysical properties and the application in automotive radiator. Renew Sustain Energy Rev. 2016;66:596–616. https://doi.org/10.1016/j.rser.2016.08.029.
Krishna VM, Kumar MS. Numerical analysis of forced convective heat transfer of nanofluids in microchannel for cooling electronic equipment. Mater Today Proc. 2019;17:295–302. https://doi.org/10.1016/j.matpr.2019.06.433.
Okonkwo EC, Okwose CF, Abid M, Ratlamwala TAH. Second-law analysis and exergoeconomics optimization of a solar tower—driven combined-cycle power plant using supercritical CO2. J Energy Eng ASCE. 2018;144(3):1–12. https://doi.org/10.1061/(ASCE)EY.1943-7897.0000534.
Okonkwo EC, Abid M, Ratlamwala TAH. Numerical analysis of heat transfer enhancement in a parabolic trough collector based on geometry modifications and working fluid usage. J Sol Energy Eng. 2018;140(5):0510091. https://doi.org/10.1115/1.4040076.
Meseguer J, Pérez-Grande I, Sanz-Andrés A. Spacecraft thermal control. 1st ed. London: Elsevier; 2012.
Sajid MU, Ali HM. Thermal conductivity of hybrid nanofluids: a critical review. Int J Heat Mass Transf. 2018;126:211–34. https://doi.org/10.1016/j.ijheatmasstransfer.2018.05.021.
Das SK, Choi SUS, Patel HE. Heat transfer in nanofluids—a review heat transfer in nanofluids. Heat Transf Eng. 2007;27(10):37–41. https://doi.org/10.1080/01457630600904593.
Kaggwa A, Carson JK. Developments and future insights of using nanofluids for heat transfer enhancements in thermal systems: a review of recent literature. Int Nano Lett. 2019;9(4):277–88. https://doi.org/10.1007/s40089-019-00281-x.
Gupta M, Singh V, Kumar R, Said Z. A review on thermophysical properties of nanofluids and heat transfer applications. Renew Sustain Energy Rev. 2017;74(March):638–70. https://doi.org/10.1016/j.rser.2017.02.073.
Ganji DD, Sabzehmeidani Y, Sedighiamiri A. Nonlinear systems in heat transfer. New York: Elsevier; 2018.
Okonkwo EC, Wole-Osho I, Kavaz D, Abid M. Comparison of experimental and theoretical methods of obtaining the thermal properties of alumina/iron mono and hybrid nanofluids. J Mol Liq. 2019;292:111377. https://doi.org/10.1016/j.molliq.2019.111377.
Choi SUS, Eastman JA. Enhancing thermal conductivity of fluids with nanoparticles. In: American Society of Mechanical Engineers, Fluids Engineering Division (Publication) FED; 1995. vol. 231, pp. 99–105.
Sivashanmugam P. Application of nanofluids in heat transfer; 2012. vol. 1.
Maxwell JC. A treatise on electricity and magnetism [volume 2]. 2nd ed. London: Clarendon Press; 1881.
Ali N, Teixeira JA, Addali A. A review on nanofluids: fabrication, stability, and thermophysical properties. J Nanomater. 2018. https://doi.org/10.1155/2018/6978130.
Okonkwo EC, Essien EA, Akhayere E, Abid M, Kavaz D, Ratlamwala TAH. Thermal performance analysis of a parabolic trough collector using water-based green-synthesized nanofluids. Sol Energy. 2018;170:658–670. https://doi.org/10.1016/j.solener.2018.06.012.
Menni Y, Chamkha AJ, Lorenzini G, Kaid N, Ameur H, Bensafi M. Advances of nanofluids in solar collectors—a review of numerical studies. Math Model Eng Probl. 2019;6(3):415–27. https://doi.org/10.18280/mmep.060313.
Wahab A, Hassan A, Qasim MA, Ali HM, Babar H, Sajid MU. Solar energy systems—potential of nanofluids. J Mol Liq. 2019;289:111049. https://doi.org/10.1016/j.molliq.2019.111049.
Ibrahim H, Sazali N, Shah ASM, Karim MSA, Aziz F, Salleh WNW. A review on factors affecting heat transfer efficiency of nanofluids for application in plate heat exchanger. J Adv Res Fluid Mech Therm Sci. 2019;60(1):144–54.
Sajid MU, Ali HM. Recent advances in application of nanofluids in heat transfer devices: a critical review. Renew Sustain Energy Rev. 2019;103:556–92. https://doi.org/10.1016/j.rser.2018.12.057.
Nazari MA, Ahmadi MH, Sadeghzadeh M, Shafii MB, Goodarzi M. A review on application of nanofluid in various types of heat pipes. J Cent South Univ. 2019;26:1021–41. https://doi.org/10.1007/s11771-019-4068-9.
Vele NS, Patil RK. Review on heat transfer enhancement in car radiator using Nano fluids. In: Proceedings of the International Conference on Industrial Engineering and Operations Management; 2019. pp. 527–537.
Ramezanizadeh M, Nazari MA, Ahmadi MH, Lorenzini G, Pop I. A review on the applications of intelligence methods in predicting thermal conductivity of nanofluids. J Therm Anal Calorim. 2019;138:827–43. https://doi.org/10.1007/s10973-019-08154-3.
Sezer N, Atieh MA, Koç M. A comprehensive review on synthesis, stability, thermophysical properties, and characterization of nanofluids. Powder Technol. 2019;344:404–31. https://doi.org/10.1016/j.powtec.2018.12.016.
Loni R, et al. Research and review study of solar dish concentrators with different nanofluids and different shapes of cavity receiver: experimental tests. Renew Energy. 2020;145:783–804. https://doi.org/10.1016/j.renene.2019.06.056.
Sahin AZ, Uddin MA, Yilbas BS, Al-Sharafi A. Performance enhancement of solar energy systems using nanofluids: an updated review. Renew Energy. 2020;145:1126–48. https://doi.org/10.1016/j.renene.2019.06.108.
Goel N, Taylor RA, Otanicar T. A review of nanofluid-based direct absorption solar collectors: design considerations and experiments with hybrid PV/Thermal and direct steam generation collectors. Renew Energy. 2020;145:903–13. https://doi.org/10.1016/j.renene.2019.06.097.
Borode A, Ahmed N, Olubambi P. A review of solar collectors using carbon-based nanofluids. J Clean Prod. 2019;241:118311. https://doi.org/10.1016/j.jclepro.2019.118311.
Abbas N, et al. Applications of nanofluids in photovoltaic thermal systems: a review of recent advances. Phys A Stat Mech Appl. 2019;536:122513. https://doi.org/10.1016/j.physa.2019.122513.
Pordanjani AH, Aghakhani S, Afrand M, Mahmoudi B, Mahian O, Wongwises S. An updated review on application of nanofluids in heat exchangers for saving energy. Energy Convers Manag. 2019;198:111886. https://doi.org/10.1016/j.enconman.2019.111886.
Ahmadi MH, Ghazvini M, Sadeghzadeh M, Nazari MA, Ghalandari M. Utilization of hybrid nanofluids in solar energy applications: a review. Nano-Struct Nano-Objects. 2019;20:100386. https://doi.org/10.1016/j.nanoso.2019.100386.
Farhana K, et al. Improvement in the performance of solar collectors with nanofluids—a state-of-the-art review. Nano-Struct Nano-Objects. 2019;18:100276. https://doi.org/10.1016/j.nanoso.2019.100276.
Borode AO, Ahmed NA, Olubambi PA. A review of heat transfer application of carbon-based nanofluid in heat exchangers. Nano-Struct Nano-Objects. 2019;20:100394. https://doi.org/10.1016/j.nanoso.2019.100394.
Nazari MA, Ghasempour R, Ahmadi MH. A review on using nanofluids in heat pipes. J Therm Anal Calorim. 2019;137(6):1847–55. https://doi.org/10.1007/s10973-019-08094-y.
Cacua K, Buitrago-Sierra R, Herrera B, Pabón E, Murshed SMS. Nanofluids’ stability effects on the thermal performance of heat pipes: a critical review. J Therm Anal Calorim. 2019;136(4):1597–614. https://doi.org/10.1007/s10973-018-7787-5.
Shah TR, Ali HM. Applications of hybrid nanofluids in solar energy, practical limitations and challenges: a critical review. Sol Energy. 2019;183:173–203. https://doi.org/10.1016/j.solener.2019.03.012.
Bumataria RK, Chavda NK, Panchal H. Current research aspects in mono and hybrid nanofluid based heat pipe technologies. Heliyon. 2019. https://doi.org/10.1016/j.heliyon.2019.e01627.
Zayed ME, Zhao J, Du Y, Kabeel AE, Shalaby SM. Factors affecting the thermal performance of the flat plate solar collector using nanofluids: a review. Sol Energy. 2019;182:382–96. https://doi.org/10.1016/j.solener.2019.02.054.
Sakhaei SA, Valipour MS. Performance enhancement analysis of The flat plate collectors: a comprehensive review. Renew Sustain Energy Rev. 2019;102:186–204. https://doi.org/10.1016/j.rser.2018.11.014.
Razali NFM, Fudholi A, Ruslan MH, Sopian K. Review of water-nanofluid based photovoltaic/thermal (PV/T) systems. Int J Electr Comput Eng. 2019;9(1):134. https://doi.org/10.11591/ijece.v9i1.
Olia H, Torabi M, Bahiraei M, Ahmadi MH, Goodarzi M, Safaei MR. Application of nanofluids in thermal performance enhancement of parabolic trough solar collector: state-of-the-art. Appl Sci. 2019;9(3):463. https://doi.org/10.3390/app9030463.
**an HW, Sidik NAC, Najafi G. Recent state of nanofluid in automobile cooling systems. J Therm Anal Calorim. 2019;135:981–1008. https://doi.org/10.1007/s10973-018-7477-3.
Rasih RA, Sidik NAC, Samion S. Numerical investigation of direct absorption solar collector using nanofluids: a review. In: IOP Conference Series: Materials Science and Engineering; 2019. https://doi.org/10.1088/1757-899x/469/1/012059.
Bellos E, Tzivanidis C. A review of concentrating solar thermal collectors with and without nanofluids. J Therm Anal Calorim. 2019;135:763–86. https://doi.org/10.1007/s10973-018-7183-1.
Ramasamy D, Sudhakara RS, Ramachandran T, Gunasekharan S. A critical review on performance of various nanofluids in solar flat plate collector, heat exchanger and radiator. Int J Mech Prod Eng Res Dev. 2020;9:74–90.
Akram N, et al. A comprehensive review on nanofluid operated solar flat plate collectors. J Therm Anal Calorim. 2019. https://doi.org/10.1007/s10973-019-08514-z.
Wang X, et al. A comprehensive review on the properties of nanofluid fuel and its additive effects to compression ignition engines. Appl Surf Sci. 2019;504(October):2019. https://doi.org/10.1016/j.apsusc.2019.144581.
Singh SK, Kumar A. An effect of twisted tape with nanofluid on the performance of double pipe heat exchanger: a comprehensive review. Int J Mech Prod Eng Res Dev. 2019;9(1):531–40. https://doi.org/10.24247/ijmperdfeb201951.
Rasih RA, Sidik NAC, Samion S. Recent progress on concentrating direct absorption solar collector using nanofluids: a review. J Therm Anal Calorim. 2019;137:903–22. https://doi.org/10.1007/s10973-018-7964-6.
Manikandan GK, Iniyan S, Goic R. Enhancing the optical and thermal efficiency of a parabolic trough collector—a review. Appl Energy. 2019;235:1524–40. https://doi.org/10.1016/j.apenergy.2018.11.048.
Sopian K, Alwaeli AHA, Kazem HA. Advanced photovoltaic thermal collectors. Proc Inst Mech Eng Part E J Process Mech Eng. 2019. https://doi.org/10.1177/0954408919869541.
Kumar A, Subudhi S. Preparation, characterization and heat transfer analysis of nanofluids used for engine cooling. Appl Therm Eng. 2019;160:114092. https://doi.org/10.1016/j.applthermaleng.2019.114092.
Qiu L, Ouyang Y, Feng Y, Zhang X. Review on micro/nano phase change materials for solar thermal applications. Renew Energy. 2019;145:650–7. https://doi.org/10.1016/j.renene.2019.03.088.
Mashali F, et al. International journal of heat and mass transfer thermo-physical properties of diamond nanofluids: a review. Int J Heat Mass Transf. 2019;129:1123–35. https://doi.org/10.1016/j.ijheatmasstransfer.2018.10.033.
Babar H. Towards hybrid nano fluids : preparation, thermophysical properties. Appl Chall. 2019;281:598–633.
Asadi A, et al. Recent advances in preparation methods and thermophysical properties of oil-based nano fluids: a state-of-the-art review. Powder Technol. 2019;352:209–26. https://doi.org/10.1016/j.powtec.2019.04.054.
Asadi A, Pourfattah F, Miklós I, Afrand M. Ultrasonics—sonochemistry effect of sonication characteristics on stability, thermophysical properties, and heat transfer of nanofluids : a comprehensive review. Ultrason Sonochem. 2019;58:104701. https://doi.org/10.1016/j.ultsonch.2019.104701.
Yu W, **e H. A review on nanofluids: preparation, stability mechanisms, and applications. J Nanomater. 2012;87:228–40. https://doi.org/10.1155/2012/435873.
Asadi A, Alarifi IM, Ali V, Nguyen HM. An experimental investigation on the effects of ultrasonication time on stability and thermal conductivity of MWCNT-water nanofluid: Finding the optimum ultrasonication time. Ultrason Sonochem. 2019;58:104639. https://doi.org/10.1016/j.ultsonch.2019.104639.
Chen W, Zou C, Li X. Application of large-scale prepared MWCNTs nanofluids in solar energy system as volumetric solar absorber. Sol Energy Mater Sol Cells. 2019;200(8):109931. https://doi.org/10.1016/j.solmat.2019.109931.
Almanassra IW, Manasrah AD, Al-Mubaiyedh UA, Al-Ansari T, Malaibari ZO, Atieh MA. An experimental study on stability and thermal conductivity of water/CNTs nanofluids using different surfactants: a comparison study. J Mol Liq. 2019. https://doi.org/10.1016/j.molliq.2019.111025.
Liu WI, et al. A novel comprehensive experimental study concerned graphene oxide nanoparticles dispersed in water: synthesise, characterisation, thermal conductivity measurement and present a new approach of RLSF neural network. Int Commun Heat Mass Transf. 2019;109:104333. https://doi.org/10.1016/j.icheatmasstransfer.2019.104333.
Aureen Albert A, Harris Samuel DG, Parthasarathy V, Kiruthiga K. A facile one pot synthesis of highly stable PVA–CuO hybrid nanofluid for heat transfer application. Chem Eng Commun. 2019. https://doi.org/10.1080/00986445.2019.1588731.
Yang L, Huang J, Ji W, Mao M. Investigations of a new combined application of nanofluids in heat recovery and air purification. Powder Technol. 2019. https://doi.org/10.1016/j.powtec.2019.10.053.
Huang J, et al. Facile preparation and thermal properties of Field’s alloy nanofluid for heat transfer. Coll Surf A Physicochem Eng Asp. 2019;581:123805. https://doi.org/10.1016/j.colsurfa.2019.123805.
Du B, Jian Q. Size controllable synthesis of graphene water nanofluid with enhanced stability. Fullerenes Nanotub Carbon Nanostruct. 2019;27(1):87–96. https://doi.org/10.1080/1536383X.2018.1529667.
Li D, Fang W, Feng Y, Geng Q, Song M. Stability properties of water-based gold and silver nanofluids stabilized by cationic gemini surfactants. J Taiwan Inst Chem Eng. 2019;97:458–65. https://doi.org/10.1016/j.jtice.2019.02.017.
Rostami S, Nadooshan AA, Raisi A. An experimental study on the thermal conductivity of new antifreeze containing copper oxide and graphene oxide nano-additives. Powder Technol. 2019;345:658–67. https://doi.org/10.1016/j.powtec.2019.01.055.
Nithiyanantham U, Grosu Y, González-Fernández L, Zaki A, Igartua JM, Faik A. Development of molten nitrate salt based nanofluids for thermal energy storage application: high thermal performance and long storage components life-time. AIP Conf Proc. 2019. https://doi.org/10.1063/1.5117740.
Mahyari AA, Karimipour A, Afrand M. Effects of dispersed added graphene oxide-silicon carbide nanoparticles to present a statistical formulation for the mixture thermal properties. Phys A Stat Mech Appl. 2019;521:98–112. https://doi.org/10.1016/j.physa.2019.01.035.
Palanisamy K, Kumar PCM. Experimental investigation on convective heat transfer and pressure drop of cone helically coiled tube heat exchanger using carbon nanotubes/water nanofluids. Heliyon. 2019;5:e01705. https://doi.org/10.1016/j.heliyon.2019.e01705.
Kiaee FM, Bahrami Z, Hormozi F. Experimental investigation on the thermal performance and new correlation for thermal conductivity of aqueous copper oxide-doped MCM-41 nanofluids. J Therm Anal Calorim. 2019. https://doi.org/10.1007/s10973-019-08832-2.
Gulzar O, Qayoum A, Gupta R. Experimental study on stability and rheological behaviour of hybrid Al2O3-TiO2 Therminol-55 nanofluids for concentrating solar collectors. Powder Technol. 2019;352:436–44. https://doi.org/10.1016/j.powtec.2019.04.060.
Prasad TR, Krishna KR, Sharma KV. Experimental testing of thermo physical properties of novel water and glycerol mixture-based silica nano fluids. Int J Recent Technol Eng. 2019;8(2):5299–305. https://doi.org/10.35940/ijrte.b1086.078219.
Bin-Abdun NA, et al. Heat transfer improvement in simulated small battery compartment using metal oxide (CuO)/deionized water nanofluid. Heat Mass Transf Stoffuebertragung. 2019. https://doi.org/10.1007/s00231-019-02719-6.
Taghizadeh A, Taghizadeh M, Azimi M, Alsagri AS, Alrobaian AA, Afrand M. Influence of cerium oxide nanoparticles on thermal conductivity of antifreeze: preparation and stability of nanofluid using surfactant. J Therm Anal Calorim. 2019. https://doi.org/10.1007/s10973-019-08422-2.
Liu J, Wang N, Song Y, Yang B. Influence of single and multiple coupling factors on the stability of paraffin-based nanofluids. Heat Mass Transf Stoffuebertragung. 2019. https://doi.org/10.1007/s00231-019-02715-w.
Rukman NSB, Fudholi A, Razali NFM, Ruslan MH, Sopian K. Investigation of TiO2 and MWCNT nanofluids-based photovoltaic-thermal (PV/T) system. IOP Conf Ser Earth Environ Sci. 2019. https://doi.org/10.1088/1755-1315/268/1/012076.
Syarif DG, Prajitno DH, Pane JS. Nanofluids with enhanced CHF prepared from self-combustion synthesized Al2O3 nanoparticles with PEG 1000 as fuel. IOP Conf Ser Mater Sci Eng. 2019. https://doi.org/10.1088/1757-899x/515/1/012046.
Kharat PB, Kounsalye JS, Shisode MV, Jadhav KM. Preparation and thermophysical investigations of CoFe2O4-based Nanofluid: a potential heat transfer agent. J Supercond Nov Magn. 2019;32(2):341–51. https://doi.org/10.1007/s10948-018-4711-y.
Wang C, Zhang X, Jia W, Deng Q, Leng Y. Preparation and tribological properties of modified field’s alloy nanoparticles as additives in liquid poly-alfa-olefin solution. J Tribol. 2019. https://doi.org/10.1115/1.4042768.
Barai DP, Bhanvase BA, Saharan VK. reduced graphene oxide-Fe3O4 nanocomposite based nanofluids: study on ultrasonic assisted synthesis, thermal conductivity, rheology, and convective heat transfer. Ind Eng Chem Res. 2019;58(19):8349–69. https://doi.org/10.1021/acs.iecr.8b05733.
Afzal A, Khan SA, Saleel CA. Role of ultrasonication duration and surfactant on characteristics of ZnO and CuO nanofluids. Mater Res Express. 2019. https://doi.org/10.1088/2053-1591/ab5013.
Nithiyanantham U, Zaki A, Grosu Y, González-Fernández L, Igartua JM, Faik A. SiO2 and Al2O3 core-shell nanoparticles based molten salts nanofluids for thermal energy storage applications. J Energy Storage. 2019;26:101033. https://doi.org/10.1016/j.est.2019.101033.
Abadeh A, Passandideh-Fard M, Maghrebi MJ, Mohammadi M. Stability and magnetization of Fe3O4/water nanofluid preparation characteristics using Taguchi method. J Therm Anal Calorim. 2019;135(2):1323–34. https://doi.org/10.1007/s10973-018-7662-4.
Mahbubul IM, Elcioglu EB, Amalina MA, Saidur R. Stability, thermophysical properties and performance assessment of alumina–water nanofluid with emphasis on ultrasonication and storage period. Powder Technol. 2019;345:668–75. https://doi.org/10.1016/j.powtec.2019.01.041.
Warjri M, Narayan J. Synthesis, characterization and physicochemical properties of cupric oxide nanoparticles and their nanofluids. Mater Today Proc. 2019;18:1176–84. https://doi.org/10.1016/j.matpr.2019.06.578.
Nazarzade S, Ghorbani HR, Jafarpourgolroudbary H. Synthesis, preparation and the experimental study of silver/water nanofluid to optimize convective heat transfer in a shell and tube heat exchanger. Inorg Nano-Metal Chem. 2019;49(6):173–6. https://doi.org/10.1080/24701556.2019.1606827.
Prasad TR, Konijeti R, Sharma KV. The experimental investigation and comparison of thermal conductivities of cobalt and silica nano fluids in glycerol water mixture as base fluid. Int J Innov Technol Explor Eng. 2019;8(7):1614–21.
Chen Z, Shahsavar A, Al-Rashed AAAA, Afrand M. The impact of sonication and stirring durations on the thermal conductivity of alumina-liquid paraffin nanofluid: an experimental assessment. Powder Technol. 2019. https://doi.org/10.1016/j.powtec.2019.11.036.
Esmaeili E, Rounaghi SA, Gruner W, Eckert J. The preparation of surfactant-free highly dispersed ethylene glycol-based aluminum nitride-carbon nanofluids for heat transfer application. Adv Powder Technol. 2019;30(10):2032–41. https://doi.org/10.1016/j.apt.2019.06.008.
Prasad TR, Krishna KR, Sharma KV. The stability and thermal conductivity of cobalt nano fluids in base liquid water and glycerol mixture. Int J Recent Technol Eng. 2019;8(3):8871–6. https://doi.org/10.35940/ijrte.c6676.098319.
Mohammed HI, Giddings D, Walker GS. Thermo-physical properties of the nano-binary fluid (acetone–zinc bromide-ZnO) as a low temperature operating fluid for use in an absorption refrigeration machine. Heat Mass Transf Stoffuebertragung. 2019. https://doi.org/10.1007/s00231-019-02760-5.
Graves JE, Latvytė E, Greenwood A, Emekwuru NG. Ultrasonic preparation, stability and thermal conductivity of a capped copper-methanol nanofluid. Ultrason Sonochem. 2019;55(January):25–31. https://doi.org/10.1016/j.ultsonch.2019.02.028.
Paul G, Chopkar M, Manna I, Das PK. Techniques for measuring the thermal conductivity of nanofluids: a review. Renew Sustain Energy Rev. 2010;14(7):2010. https://doi.org/10.1016/j.rser.2010.03.017.
Healy JJ, De Groot JJ, Kestin J. The theory of the transient hot-wire method for measuring thermal conductivity. Phys B + C. 1976;82(2):392–408. https://doi.org/10.1016/0378-4363(76)90203-5.
Einstein A. Paper 1. A new determination of molecular dimensions. Zurich: University of Zurich; 1905.
Koca HD, Doganay S, Turgut A, Tavman IH, Saidur R, Mahbubul IM. Effect of particle size on the viscosity of nanofluids: a review. Renew Sustain Energy Rev. 2018. https://doi.org/10.1016/j.rser.2017.07.016.
Mooney M. The viscosity of a concentrated suspension of spherical particles. J Colloid Sci. 1951. https://doi.org/10.1016/0095-8522(51)90036-0.
Krieger IM, Dougherty TJ. A mechanism for non-newtonian flow in suspensions of rigid spheres. Trans Soc Rheol. 1959. https://doi.org/10.1122/1.548848.
Nielsen LE. Generalized equation for the elastic moduli of composite materials. J Appl Phys. 1970. https://doi.org/10.1063/1.1658506.
Batchelor GK. The effect of Brownian motion on the bulk stress in a suspension of spherical particles. J Fluid Mech. 1977;83(01):97. https://doi.org/10.1017/S0022112077001062.
Bruggeman VDAG. Berechnung verschiedener physikalischer Konstanten von heterogenen Substanzen. Ann Phys. 1935;5(24):636–64. https://doi.org/10.1002/andp.19354160705.
Hamilton RL, Crosser OK. Thermal conductivity of heterogeneous two-component systems. Ind Eng Chem Fundam. 1962;1(3):187–91. https://doi.org/10.1021/i160003a005.
Mahian O, et al. Recent advances in modeling and simulation of nanofluid flows—part II: applications. Phys Rep. 2019;791:1–59. https://doi.org/10.1016/j.physrep.2018.11.003.
Dadhich M, Prajapati OS, Rohatgi N. Flow boiling heat transfer analysis of Al2O3 and TiO2 nanofluids in horizontal tube using artificial neural network (ANN). J Therm Anal Calorim. 2020;139(5):3197–217. https://doi.org/10.1007/s10973-019-08674-y.
Yang L, Ji W, Mao M, Huang J. Dynamic stability, sedimentation, and time-dependent heat transfer characteristics of TiO2 and CNT nanofluids. J Therm Anal Calorim. 2019. https://doi.org/10.1007/s10973-019-09103-w.
Hameed MS, Suresh S, Singh RK. Comparative study of heat transfer and friction characteristics of water-based Alumina–copper and Alumina–CNT hybrid nanofluids in laminar flow through pipes. J Therm Anal Calorim. 2019;136(1):243–53. https://doi.org/10.1007/s10973-018-7898-z.
Yu W, France DM, Routbort JL, Choi SUS. Review and comparison of nanofluid thermal conductivity and heat transfer enhancements. Heat Transf Eng. 2008;29(5):432–60. https://doi.org/10.1080/01457630701850851.
Esfe MH, Afrand M. An updated review on the nanofluids characteristics. J Therm Anal Calorim. 2019;138(6):4091–101. https://doi.org/10.1007/s10973-019-08406-2.
Akhgar A, Toghraie D, Sina N, Afrand M. Develo** dissimilar artificial neural networks (ANNs) to prediction the thermal conductivity of MWCNT-TiO2/Water-ethylene glycol hybrid nano fluid. Powder Technol. 2019;355:602–10. https://doi.org/10.1016/j.powtec.2019.07.086.
Alarifi IM, Nguyen HM, Bakhtiyari AN, Asadi A. Feasibility of ANFIS-PSO and ANFIS-GA models in predicting thermophysical properties of Al2O3-MWCNT/Oil hybrid nanofluid. Materials (Basel). 2019;12(21):3628. https://doi.org/10.3390/ma12213628.
Moldoveanu GM, Minea AA, Huminic G, Huminic A. Al2O3/TiO2 hybrid nanofluids thermal conductivity. J Therm Anal Calorim. 2019;137(2):583–92. https://doi.org/10.1007/s10973-018-7974-4.
Essajai R, Tabtab I, Mzerd A, Mounkachi O, Hassanain N, Qjani M. Molecular dynamics study of thermal properties of nanofluids composed of one-dimensional (1-D) network of interconnected gold nanoparticles. Results Phys. 2019;15(2019):102576. https://doi.org/10.1016/j.rinp.2019.102576.
Rehman WU, et al. Synthesis, characterization, stability and thermal conductivity of multi-walled carbon nanotubes (MWCNTs) and eco-friendly jatropha seed oil based nanofluid: an experimental investigation and modeling approach. J Mol Liq. 2019;293(2019):111534. https://doi.org/10.1016/j.molliq.2019.111534.
Khalifeh A, Vaferi B. Intelligent assessment of effect of aggregation on thermal conductivity of nanofluids—comparison by experimental data and empirical correlations. Thermochim Acta. 2019;681(2019):178377. https://doi.org/10.1016/j.tca.2019.178377.
Mirsaeidi AM, Yousefi F. Viscosity, thermal conductivity and density of carbon quantum dots nanofluids: an experimental investigation and development of new correlation function and ANN modeling. J Therm Anal Calorim. 2019. https://doi.org/10.1007/s10973-019-09138-z.
Motlagh SY, Sharifi A, Ahmadi M, Badfar H. Presentation of new thermal conductivity expression for Al2O3–water and CuO–water nanofluids using gene expression programming (GEP). J Therm Anal Calorim. 2019;135(1):195–206. https://doi.org/10.1007/s10973-018-7305-9.
Mousavi SM, Esmaeilzadeh F, Wang XP. Effects of temperature and particles volume concentration on the thermophysical properties and the rheological behavior of CuO/MgO/TiO2 aqueous ternary hybrid nanofluid: experimental investigation. J Therm Anal Calorim. 2019;137(3):879–901. https://doi.org/10.1007/s10973-019-08006-0.
Taherialekouhi R, Rasouli S, Khosravi A. An experimental study on stability and thermal conductivity of water-graphene oxide/aluminum oxide nanoparticles as a cooling hybrid nanofluid. Int J Heat Mass Transf. 2019;145(2019):118751. https://doi.org/10.1016/j.ijheatmasstransfer.2019.118751.
Akilu S, Baheta AT, Chowdhury S, Padmanabhan E, Sharma KV. Thermophysical profile of SiC–CuO/C nanocomposite in base liquid ethylene glycol. Powder Technol. 2019;354(2019):540–51. https://doi.org/10.1016/j.powtec.2019.04.061.
Shahsavar A, Godini A, Sardari PT, Toghraie D, Salehipour H. Impact of variable fluid properties on forced convection of Fe3O4/CNT/water hybrid nanofluid in a double-pipe mini-channel heat exchanger. J Therm Anal Calorim. 2019;137(3):1031–43. https://doi.org/10.1007/s10973-018-07997-6.
Arani AAA, Pourmoghadam F. Experimental investigation of thermal conductivity behavior of MWCNTS-Al2O3/ethylene glycol hybrid Nanofluid: providing new thermal conductivity correlation. Heat Mass Transf Stoffuebertragung. 2019;55(8):2329–39. https://doi.org/10.1007/s00231-019-02572-7.
Khan AI, et al. Experimental investigation of thermal conductivity and stability of TiO2-Ag/water nanocomposite fluid with SDBS and SDS surfactants. Thermochim Acta. 2019;678:178308. https://doi.org/10.1016/j.tca.2019.178308.
Rubasingh BJ, Selvakumar P, Raja RSS. Predicting thermal conductivity behaviour of ZnO, TiO2 and ball milled TiO2/ZnO based nanofluids with ethylene glycol as base fluid. Mater Res Express. 2019;6(9):095702. https://doi.org/10.1088/2053-1591/ab2bc5.
Sulgani MT, Karimipour A. Improve the thermal conductivity of 10w40-engine oil at various temperature by addition of Al2O3/Fe2O3 nanoparticles. J Mol Liq. 2019;283:660–6. https://doi.org/10.1016/j.molliq.2019.03.140.
Mousavi SM, Esmaeilzadeh F, Wang XP. A detailed investigation on the thermo-physical and rheological behavior of MgO/TiO 2 aqueous dual hybrid nanofluid. J Mol Liq. 2019;282:323–39. https://doi.org/10.1016/j.molliq.2019.02.100.
De Oliveira LR, Ribeiro SRFL, Reis MHM, Cardoso VL, Filho EPB. Experimental study on the thermal conductivity and viscosity of ethylene glycol-based nanofluid containing diamond-silver hybrid material. Diam Relat Mater. 2019;96:216–30. https://doi.org/10.1016/j.diamond.2019.05.004.
Wole-Osho I, Okonkwo EC, Adun H, Kavaz D, Abbasoglu S. An intelligent approach to predicting the effect of nanoparticle mixture ratio, concentration, and temperature on thermal conductivity of hybrid nanofluids. J Therm Anal Calorim. 2020;15:459. https://doi.org/10.1007/s10973-020-09594-y
Ahmed W, et al. Experimental investigation of convective heat transfer growth on ZnO@TiO2/DW binary composites/hybrid nanofluids in a circular heat exchanger. J Therm Anal Calorim. 2020. https://doi.org/10.1007/s10973-020-09363-x.
Giwa SO, Sharifpur M, Goodarzi M, Alsulami H, Meyer JP. Influence of base fluid, temperature, and concentration on the thermophysical properties of hybrid nanofluids of alumina–ferrofluid: experimental data, modeling through enhanced ANN, ANFIS, and curve fitting. J Therm Anal Calorim. 2020. https://doi.org/10.1007/s10973-020-09372-w.
Pourrajab R, Noghrehabadi A, Behbahani M, Hajidavalloo E. An efficient enhancement in thermal conductivity of water-based hybrid nanofluid containing MWCNTs-COOH and Ag nanoparticles: experimental study. J Therm Anal Calorim. 2020. https://doi.org/10.1007/s10973-020-09300-y.
Izadkhah MS, Heris SZ. Influence of Al2O3 nanoparticles on the stability and viscosity of nanofluids: Insights from molecular dynamics simulation. J Therm Anal Calorim. 2019;138(1):623–31. https://doi.org/10.1007/s10973-019-08228-2.
Dehghani Y, Abdollahi A, Karimipour A. Experimental investigation toward obtaining a new correlation for viscosity of WO3 and Al2O3 nanoparticles-loaded nanofluid within aqueous and non-aqueous basefluids. J Therm Anal Calorim. 2019;135(1):713–28. https://doi.org/10.1007/s10973-018-7394-5.
Ye X, Kandlikar SG, Li C. Viscosity of nanofluids containing anisotropic particles: a critical review and a comprehensive model. Eur Phys J E. 2019;42(12):60–5. https://doi.org/10.1140/epje/i2019-11923-7.
Bahrami M, Akbari M, Bagherzadeh SA, Karimipour A, Afrand M, Goodarzi M. Develop 24 dissimilar ANNs by suitable architectures & training algorithms via sensitivity analysis to better statistical presentation: measure MSEs between targets and ANN for Fe–CuO/Eg–Water nanofluid. Phys A Stat Mech Appl. 2019;519(2019):159–68. https://doi.org/10.1016/j.physa.2018.12.031.
Ruhani B, Toghraie D, Hekmatifar M, Hadian M. Statistical investigation for develo** a new model for rheological behavior of ZnO–Ag (50%–50%)/Water hybrid Newtonian nanofluid using experimental data. Phys A Stat Mech Appl. 2019;525(2019):741–51. https://doi.org/10.1016/j.physa.2019.03.118.
Mousavi SB, Heris SZ, Hosseini MG. Experimental investigation of MoS2/diesel oil nanofluid thermophysical and rheological properties. Int Commun Heat Mass Transf. 2019;108:104298. https://doi.org/10.1016/j.icheatmasstransfer.2019.104298.
Hameed A, et al. Experimental investigation on synthesis, characterization, stability, thermo-physical properties and rheological behavior of MWCNTs-kapok seed oil based nanofluid. J Mol Liq. 2019;277(2019):812–24. https://doi.org/10.1016/j.molliq.2019.01.012.
Aghahadi MH, Niknejadi M, Toghraie D. An experimental study on the rheological behavior of hybrid Tungsten oxide (WO3)-MWCNTs/engine oil Newtonian nanofluids. J Mol Struct. 2019;1197(2019):497–507. https://doi.org/10.1016/j.molstruc.2019.07.080.
Alarifi IM, Alkouh AB, Ali V, Nguyen HM, Asadi A. On the rheological properties of MWCNT-TiO2/oil hybrid nanofluid: an experimental investigation on the effects of shear rate, temperature, and solid concentration of nanoparticles. Powder Technol. 2019;355(2019):157–62. https://doi.org/10.1016/j.powtec.2019.07.039.
Esfe MH, Abad ATK, Fouladi M. Effect of suspending optimized ratio of nano-additives MWCNT-Al2O3 on viscosity behavior of 5W50. J Mol Liq. 2019;285:572–85. https://doi.org/10.1016/j.molliq.2019.04.043.
Esfe MH, Esfandeh S, Niazi S. An experimental investigation, sensitivity analysis and RSM analysis of MWCNT(10)-ZnO(90)/10W40 nanofluid viscosity. J Mol Liq. 2019;288:111020. https://doi.org/10.1016/j.molliq.2019.111020.
Esfe MH, Emami MRS, Amiri MK. Experimental investigation of effective parameters on MWCNT–TiO2/SAE50 hybrid nanofluid viscosity. J Therm Anal Calorim. 2019;137(3):743–57. https://doi.org/10.1007/s10973-018-7986-0.
Goodarzi M, Toghraie D, Reiszadeh M, Afrand M. Experimental evaluation of dynamic viscosity of ZnO–MWCNTs/engine oil hybrid nanolubricant based on changes in temperature and concentration. J Therm Anal Calorim. 2019;136(2019):513–25. https://doi.org/10.1007/s10973-018-7707-8.
Kumar V, Sahoo RR. Viscosity and thermal conductivity comparative study for hybrid nanofluid in binary base fluids. Heat Transf Asian Res. 2019;48(7):3144–61. https://doi.org/10.1002/htj.21535.
Talebizadehsardari P, Shahsavar A, Toghraie D, Barnoon P. An experimental investigation for study the rheological behavior of water–carbon nanotube/magnetite nanofluid subjected to a magnetic field. Phys A Stat Mech Appl. 2019;534(2019):122129. https://doi.org/10.1016/j.physa.2019.122129.
Vallejo JP, Sani E, Żyła G, Lugo L. Tailored silver/graphene nanoplatelet hybrid nanofluids for solar applications. J Mol Liq. 2019;296(2019):112007. https://doi.org/10.1016/j.molliq.2019.112007.
Wole-Osho I, Okonkwo EC, Kavaz D, Abbasoglu S. An experimental investigation into the effect of particle mixture ratio on specific heat capacity and dynamic viscosity of Al2O3-ZnO hybrid nanofluids. Powder Technol. 2020;363:699–716. https://doi.org/10.1016/j.powtec.2020.01.015.
Moldoveanu GM, Minea AA. Specific heat experimental tests of simple and hybrid oxide-water nanofluids: proposing new correlation. J Mol Liq. 2019;279:299–305. https://doi.org/10.1016/j.molliq.2019.01.137.
Li Y, et al. Experimental study on the effect of SiO2 nanoparticle dispersion on the thermophysical properties of binary nitrate molten salt. Sol Energy. 2019;183(2019):776–81. https://doi.org/10.1016/j.solener.2019.03.036.
Hassan MA, Banerjee D. A soft computing approach for estimating the specific heat capacity of molten salt-based nanofluids. J Mol Liq. 2019;281(2019):365–75. https://doi.org/10.1016/j.molliq.2019.02.106.
Alade IO, Rahman MAA, Saleh TA. Predicting the specific heat capacity of alumina/ethylene glycol nanofluids using support vector regression model optimized with Bayesian algorithm. Sol Energy. 2019;183:74–82. https://doi.org/10.1016/j.solener.2019.02.060.
Marcos MA, et al. MWCNT in PEG-400 nanofluids for thermal applications: a chemical, physical and thermal approach. J Mol Liq. 2019;294:111616. https://doi.org/10.1016/j.molliq.2019.111616.
Qiu L, et al. A review of recent advances in thermophysical properties at the nanoscale: from solid state to colloids. Phys Rep. 2020;843:1–81. https://doi.org/10.1016/j.physrep.2019.12.001.
Xuan Y, Li WHQ. Aggregation structure and thermal conductivity of nanofluids. AIChE J. 2003;49(4):1038–43.
Lee JH, et al. Effective viscosities and thermal conductivities of aqueous nanofluids containing low volume concentrations of Al2O3 nanoparticles. Int J Heat Mass Transf. 2008;51:2651–6. https://doi.org/10.1016/j.ijheatmasstransfer.2007.10.026.
Afzal A, Nawfal I, Mahbubul IM, Kumbar SS. An overview on the effect of ultrasonication duration on different properties of nanofluids. J Therm Anal Calorim. 2019;135(1):393–418. https://doi.org/10.1007/s10973-018-7144-8.
Cacua K, Murshed SMS, Pabón E, Buitrago R. Dispersion and thermal conductivity of TiO2/water nanofluid: effects of ultrasonication, agitation and temperature. J Therm Anal Calorim. 2020;140(1):109–14. https://doi.org/10.1007/s10973-019-08817-1.
Wang J, Li G, Li T, Zeng M, Sundén B. Effect of various surfactants on stability and thermophysical properties of nanofluids. J Therm Anal Calorim. 2020. https://doi.org/10.1007/s10973-020-09381-9.
Cakmak NK. The impact of surfactants on the stability and thermal conductivity of graphene oxide de-ionized water nanofluids. J Therm Anal Calorim. 2020;139(3):1895–902. https://doi.org/10.1007/s10973-019-09096-6.
Asadi A, et al. Effect of sonication characteristics on stability, thermophysical properties, and heat transfer of nanofluids: a comprehensive review. Ultrason Sonochem. 2019;58(2019):104701. https://doi.org/10.1016/j.ultsonch.2019.104701.
Khan AI, Arasu AV. A review of influence of nanoparticle synthesis and geometrical parameters on thermophysical properties and stability of nanofluids. Therm Sci Eng Progress. 2019. https://doi.org/10.1016/j.tsep.2019.04.010.
Yang L, Ji W, Huang JN, Xu G. An updated review on the influential parameters on thermal conductivity of nano-fluids. J Mol Liq. 2019;296:111780. https://doi.org/10.1016/j.molliq.2019.111780.
Yang L, Ji W, Zhang Z, ** X. Thermal conductivity enhancement of water by adding graphene Nano-sheets: consideration of particle loading and temperature effects. Int Commun Heat Mass Transf. 2019;109:104353. https://doi.org/10.1016/j.icheatmasstransfer.2019.104353.
Yang L, Mao M, Huang JN, Ji W. Enhancing the thermal conductivity of SAE 50 engine oil by adding zinc oxide nano-powder: an experimental study. Powder Technol. 2019;356:335–41. https://doi.org/10.1016/j.powtec.2019.08.031.
Mahian O, et al. Recent advances in modeling and simulation of nanofluid flows-part I: fundamentals and theory. Phys Rep. 2019;790:1–48. https://doi.org/10.1016/j.physrep.2018.11.004.
Jang SP, Choi SUS. Effects of various parameters on nanofluid thermal conductivity. J Heat Transfer. 2013;129(5):617–23. https://doi.org/10.1115/1.2712475.
Keblinski P, Phillpot SR, Choi SUS, Eastman JA. Mechanism of heat flow in suspensions of nano-sized particles (nanofluids). Int J Heat Mass Transf. 2002;45:855–63.
Yu W, Choi SUS. The role of interfacial layers in the enhanced thermal conductivity of nanofluids: a renovated Maxwell model. J Nanoparticle Res. 2003;5:167–71.
**e H, Fujii M, Zhang X. Effect of interfacial nanolayer on the effective thermal conductivity of nanoparticle-fluid mixture. Int J Heat Mass Transf. 2005;48:2926–32. https://doi.org/10.1016/j.ijheatmasstransfer.2004.10.040.
Pinto RV, Augusto F, Fiorelli S. Review of the mechanisms responsible for heat transfer enhancement using nanofluids. Appl Therm Eng. 2016;108:720–39. https://doi.org/10.1016/j.applthermaleng.2016.07.147.
Khalid M, Rahman S, Ong SS, Saidur R. Preparation, thermo-physical properties and heat transfer enhancement of nanofluids. Mater Res Express. 2014;1(3):032001. https://doi.org/10.1088/2053-1591/1/3/032001.
Chandrasekar M, Suresh S. A review on the mechanisms of heat transport in nanofluids. Heat Transf Eng. 2011;30(14):1136–50. https://doi.org/10.1080/01457630902972744.
Wong KV, Castillo MJ. Heat transfer mechanisms and clustering in nanofluids. Adv Mech Eng. 2010. https://doi.org/10.1155/2010/795478.
Wang GCJJ, Zheng RT, Gao JW. Heat conduction mechanisms in nanofluids and suspensions. Nano Today. 2012;7:124–36.
Choudhary S, Sachdeva A, Kumar P. Investigation of the stability of MgO nanofluid and its effect on the thermal performance of flat plate solar collector. Renew Energy. 2020;147:1801–14. https://doi.org/10.1016/j.renene.2019.09.126.
Ahmadlouydarab M, Ebadolahzadeh M, Ali HM. Effects of utilizing nanofluid as working fluid in a lab-scale designed FPSC to improve thermal absorption and efficiency. Phys A Stat Mech Appl. 2020;540:123109. https://doi.org/10.1016/j.physa.2019.123109.
Saffarian MR, Moravej M, Doranehgard MH. Heat transfer enhancement in a flat plate solar collector with different flow path shapes using nanofluid. Renew Energy. 2020;146:2316–29. https://doi.org/10.1016/j.renene.2019.08.081.
Tong Y, Lee H, Kang W, Cho H. Energy and exergy comparison of a flat-plate solar collector using water, Al2O3 nanofluid, and CuO nanofluid. Appl Therm Eng. 2019;159:113959. https://doi.org/10.1016/j.applthermaleng.2019.113959.
Mondragon R, Sanchez D, Cabello R, Llopis R, Julia E. Flat plate solar collector performance using alumina nanofluids: experimental characterization and efficiency tests. PLoS ONE. 2019;14(2):e0212260.
Sarafraz MM, Tlili I, Baseer MA, Safaei MR. Potential of solar collectors for clean thermal energy production in smart cities using nanofluids: experimental assessment and efficiency improvement. Appl Sci. 2019;9(9):1877. https://doi.org/10.3390/app9091877.
Natividade PSG, De Moura GM, Avallone E, Filho EPB, Gelamo RV, De Gonçalves JCSI. Experimental analysis applied to an evacuated tube solar collector equipped with parabolic concentrator using multilayer graphene-based nanofluids. Renew Energy. 2019;138:152–60. https://doi.org/10.1016/j.renene.2019.01.091.
Sadeghi G, Nazari S, Ameri M, Shama F. Energy and exergy evaluation of the evacuated tube solar collector using Cu2O/water nanofluid utilizing ANN methods. Sustain Energy Technol Assess. 2020;37:100578. https://doi.org/10.1016/j.seta.2019.100578.
Korres D, Bellos E, Tzivanidis C. Investigation of a nanofluid-based compound parabolic trough solar collector under laminar flow conditions. Appl Therm Eng. 2019;149:366–76. https://doi.org/10.1016/j.applthermaleng.2018.12.077.
Kalogirou SA. Solar energy engineering: processes and systems. 2nd ed. Springer: Berlin; 2014.
Ghodbane M, Said Z, Hachicha AA, Boumeddane B. Performance assessment of linear Fresnel solar reflector using MWCNTs/DW nanofluids. Renew Energy. 2019. https://doi.org/10.1016/j.renene.2019.10.137.
Okonkwo EC, Essien EA, Kavaz D, Abid M, Ratlamwala TAH. Olive leaf synthesized nanofluids for solar parabolic trough collector-thermal performance evaluation. J Therm Sci Eng Appl. 2019;11(4):041009. https://doi.org/10.1115/1.4043820.
Ehyaei MA, Ahmadi A, Assad MEH, Hachicha AA, Said Z. Energy, exergy and economic analyses for the selection of working fluid and metal oxide nanofluids in a parabolic trough collector. Sol Energy. 2019;187:175–84. https://doi.org/10.1016/j.solener.2019.05.046.
Malekan M, Khosravi A, Syri S. Heat transfer modeling of a parabolic trough solar collector with working fluid of Fe3O4 and CuO/Therminol 66 nanofluids under magnetic field. Appl Therm Eng. 2019;163:114435. https://doi.org/10.1016/j.applthermaleng.2019.114435.
Bellos E, Tzivanidis C. Thermal efficiency enhancement of nanofluid-based parabolic trough collectors. J Therm Anal Calorim. 2019;135(1):597–608. https://doi.org/10.1007/s10973-018-7056-7.
Qin C, Kim JB, Lee BJ. Performance analysis of a direct-absorption parabolic-trough solar collector using plasmonic nanofluids. Renew Energy. 2019;143:24–33. https://doi.org/10.1016/j.renene.2019.04.146.
Tafarroj MM, Daneshazarian R, Kasaeian A. CFD modeling and predicting the performance of direct absorption of nanofluids in trough collector. Appl Therm Eng. 2019;148:256–69. https://doi.org/10.1016/j.applthermaleng.2018.11.020.
Simonetti M, Restagno F, Sani E, Noussan M. Numerical investigation of direct absorption solar collectors (DASC), based on carbon-nanohorn nanofluids, for low temperature applications. Sol Energy. 2020;195:166–75. https://doi.org/10.1016/j.solener.2019.11.044.
Sangeetha M, Manigandan S, Chaichan MT, Kumar V. Progress of MWCNT, Al2O3, and CuO with water in enhancing the photovoltaic thermal system. Int J Energy Res. 2019. https://doi.org/10.1002/er.4905.
Alous S, Kayfeci M, Uysal A. Experimental investigations of using MWCNTs and graphene nanoplatelets water-based nanofluids as coolants in PVT systems. Appl Therm Eng. 2019;162:114265. https://doi.org/10.1016/j.applthermaleng.2019.114265.
Fudholi A, et al. TiO2/water-based photovoltaic thermal (PVT) collector: novel theoretical approach. Energy. 2019;183:305–14. https://doi.org/10.1016/j.energy.2019.06.143.
Abdelrazik AS, Al-Sulaiman FA, Saidur R, Ben-Mansour R. Evaluation of the effects of optical filtration and nanoPCM on the performance of a hybrid photovoltaic-thermal solar collector. Energy Convers Manag. 2019;195:139–56. https://doi.org/10.1016/j.enconman.2019.04.083.
AL-Musawi AIA, Taheri A, Farzanehnia A, Sardarabadi M, Passandideh-Fard M. Numerical study of the effects of nanofluids and phase-change materials in photovoltaic thermal (PVT) systems. J Therm Anal Calorim. 2019;137(2):623–36. https://doi.org/10.1007/s10973-018-7972-6.
Gulzar O, Qayoum A, Gupta R. Photo-thermal characteristics of hybrid nanofluids based on Therminol-55 oil for concentrating solar collectors. Appl Nanosci. 2019;9(5):1133–43. https://doi.org/10.1007/s13204-018-0738-4.
Qu J, Zhang R, Wang Z, Wang Q. Photo-thermal conversion properties of hybrid CuO-MWCNT/H2O nanofluids for direct solar thermal energy harvest. Appl Therm Eng. 2019;147:390–8. https://doi.org/10.1016/j.applthermaleng.2018.10.094.
Hazra SK, Ghosh S, Nandi TK. Photo-thermal conversion characteristics of carbon black-ethylene glycol nanofluids for applications in direct absorption solar collectors. Appl Therm Eng. 2019. https://doi.org/10.1016/j.applthermaleng.2019.114402.
Motamedi M, et al. Experimental testing of hydrophobic microchannels, with and without nanofluids, for solar PV/T collectors. Energies. 2019. https://doi.org/10.3390/en12153036.
Du Q, Xu J, Cheng Z, Gao J. Experimental study on sunlight absorption characteristics of Au-CuS blended nanofluids. Energy Sources Part A Recover Util Environ Eff. 2019. https://doi.org/10.1080/15567036.2019.1576078.
Wang D, et al. Enhanced photothermal conversion properties of magnetic nanofluids through rotating magnetic field for direct absorption solar collector. J Colloid Interface Sci. 2019;557:266–75. https://doi.org/10.1016/j.jcis.2019.09.022.
Wang K, et al. Significant photothermal conversion enhancement of nanofluids induced by Rayleigh-Bénard convection for direct absorption solar collectors. Appl Energy. 2019;254:20. https://doi.org/10.1016/j.apenergy.2019.113706.
Sharaf OZ, et al. Ultrastable plasmonic nanofluids in optimized direct absorption solar collectors. Energy Convers Manag. 2019;199:112010. https://doi.org/10.1016/j.enconman.2019.112010.
Liu C, Wang D, He Y, Wang K, Yu W. Properties of solar energy absorption and photothermal conversion at medium temperature based on magnetic nanofluids. Kexue Tongbao/Chinese Sci Bull. 2019;64(28–29):3041–8. https://doi.org/10.1360/TB-2019-0186.
Vallejo JP, et al. Comparative study of different functionalized graphene-nanoplatelet aqueous nanofluids for solar energy applications. Renew Energy. 2019;141:791–801. https://doi.org/10.1016/j.renene.2019.04.075.
Sami S. Impact of magnetic field on the enhancement of performance of thermal solar collectors using nanofluids. Int J Ambient Energy. 2019;40(8):875–84. https://doi.org/10.1080/01430750.2018.1437561.
Farshad SA, Sheikholeslami M. Nanofluid flow inside a solar collector utilizing twisted tape considering exergy and entropy analysis. Renew Energy. 2019;141:246–58. https://doi.org/10.1016/j.renene.2019.04.007.
Delfani S, Esmaeili M, Karami M. Application of artificial neural network for performance prediction of a nanofluid-based direct absorption solar collector. Sustain Energy Technol Assess. 2019;36:100559. https://doi.org/10.1016/j.seta.2019.100559.
Sadeghzadeh M, Ahmadi MH, Kahani M, Sakhaeinia H, Chaji H, Chen L. Smart modeling by using artificial intelligent techniques on thermal performance of flat-plate solar collector using nanofluid. Energy Sci Eng. 2019;7(5):1649–58. https://doi.org/10.1002/ese3.381.
Okonkwo EC, Adun H, Babatunde AA, Abid M, Ratlamwala TAH. Entropy generation minimization in a parabolic trough collector operating with SiO2—water nanofluids using genetic algorithm and artificial neural network. J Therm Sci Eng Appl. 2020;12(3):031007. https://doi.org/10.1115/1.4044755.
Sharafeldin MA, Gróf G. Efficiency of evacuated tube solar collector using WO3/Water nanofluid. Renew Energy. 2019;134:453–60. https://doi.org/10.1016/j.renene.2018.11.010.
Peng Y, et al. Investigation of energy performance in a U-shaped evacuated solar tube collector using oxide added nanoparticles through the emitter, absorber and transmittal environments via discrete ordinates radiation method. J Therm Anal Calorim. 2019. https://doi.org/10.1007/s10973-019-08684-w.
Kaya H, Arslan K. Numerical investigation of efficiency and economic analysis of an evacuated U-tube solar collector with different nanofluids. Heat Mass Transf Stoffuebertragung. 2019;55(3):581–93. https://doi.org/10.1007/s00231-018-2442-z.
Kang W, Shin Y, Cho H. Experimental investigation on the heat transfer performance of evacuated tube solar collector using CuO nanofluid and water. J Mech Sci Technol. 2019;33(3):1477–85. https://doi.org/10.1007/s12206-019-0249-6.
Sharafeldin MA, Gróf G, Abu-Nada E, Mahian O. Evacuated tube solar collector performance using copper nanofluid: energy and environmental analysis. Appl Therm Eng. 2019;162:114205. https://doi.org/10.1016/j.applthermaleng.2019.114205.
Sarafraz MM, Safaei MR. Diurnal thermal evaluation of an evacuated tube solar collector (ETSC) charged with graphene nanoplatelets-methanol nano-suspension. Renew Energy. 2019;142:364–72. https://doi.org/10.1016/j.renene.2019.04.091.
Mercan M, Yurddaş A. Numerical analysis of evacuated tube solar collectors using nanofluids. Sol Energy. 2019;191:167–79. https://doi.org/10.1016/j.solener.2019.08.074.
Sadeghi G, Safarzadeh H, Ameri M. Experimental and numerical investigations on performance of evacuated tube solar collectors with parabolic concentrator, applying synthesized Cu2O/distilled water nanofluid. Energy Sustain Dev. 2019;48:88–106. https://doi.org/10.1016/j.esd.2018.10.008.
Bellos E, Tzivanidis C, Papadopoulos A. Enhancing the performance of a linear Fresnel reflector using nanofluids and internal finned absorber. J Therm Anal Calorim. 2019;135(1):237–55. https://doi.org/10.1007/s10973-018-6989-1.
Arora S, Fekadu G, Subudhi S. Energy and exergy analysis of marquise shaped channel flat plate solar collector using Al2O3-water nanofluid and water. J Sol Energy Eng Trans ASME. 2019. https://doi.org/10.1115/1.4042454.
Sacithra A, Manivannan A. Turbulent flow analysis of a flattened tube in- plane curved solar collector using Titanium oxide nanofluid. Heat Mass Transf Stoffuebertragung. 2019;55(6):1783–99. https://doi.org/10.1007/s00231-018-02557-y.
Farhana K, Kadirgama K, Noor MM, Rahman MM, Ramasamy D, Mahamude ASF. CFD modelling of different properties of nanofluids in header and riser tube of flat plate solar collector. In: IOP Conference Series: Materials Science and Engineering; 2019. https://doi.org/10.1088/1757-899x/469/1/012041.
Stalin PMJ, Arjunan TV, Matheswaran MM, Sadanandam N. Experimental and theoretical investigation on the effects of lower concentration CeO2/water nanofluid in flat-plate solar collector. J Therm Anal Calorim. 2019;135(1):29–44. https://doi.org/10.1007/s10973-017-6865-4.
Kabeel AE, El-Agouz ES, Prakash N, Prasad C, Sathyamurthy R, Manokar AM. Performance analysis of spiral and serpentine tube solar collector with carbon nanotube nanofluids under natural flow method. Heat Transf Asian Res. 2019;48(6):2428–39. https://doi.org/10.1002/htj.21502.
Hajabdollahi Z, Hajabdollahi H, Kim KC. Multi-objective optimization of solar collector using water-based nanofluids with different types of nanoparticles. J Therm Anal Calorim. 2019. https://doi.org/10.1007/s10973-019-08444-w.
Hajabdollahi Z, Mirzaei M, Kim KC. Effects of a mixture of cuo and al2o3 nanoparticles on the thermal efficiency of a flat plate solar collector at different mass flow rates. Heat Transf Res. 2019;50(10):945–65. https://doi.org/10.1615/HeatTransRes.2018027822.
Stalin PMJ, Arjunan TV, Matheswaran MM, Dolli H, Sadanandam N. Energy, economic and environmental investigation of a flat plate solar collector with CeO2/water nanofluid. J Therm Anal Calorim. 2019. https://doi.org/10.1007/s10973-019-08670-2.
Mirzaei M. Experimental investigation of CuO nanofluid in the thermal characteristics of a flat plate solar collector. Environ Prog Sustain Energy. 2019;38(1):260–7. https://doi.org/10.1002/ep.12902.
Eltaweel M, Abdel-Rehim AA. Energy and exergy analysis of a thermosiphon and forced-circulation flat-plate solar collector using MWCNT/Water nanofluid. Case Stud Therm Eng. 2019;14:100416. https://doi.org/10.1016/j.csite.2019.100416.
Akram N, et al. An experimental investigation on the performance of a flat-plate solar collector using eco-friendly treated graphene nanoplatelets–water nanofluids. J Therm Anal Calorim. 2019;138(1):609–21. https://doi.org/10.1007/s10973-019-08153-4.
Alawi OA, Kamar HM, Mallah AR, Kazi SN, Sidik NAC. Thermal efficiency of a flat-plate solar collector filled with Pentaethylene Glycol-Treated Graphene Nanoplatelets: an experimental analysis. Sol Energy. 2019;191:360–70. https://doi.org/10.1016/j.solener.2019.09.011.
Jouybari HJ, Nimvari ME, Saedodin S. Thermal performance evaluation of a nanofluid-based flat-plate solar collector: an experimental study and analytical modeling. J Therm Anal Calorim. 2019;137(5):1757–74. https://doi.org/10.1007/s10973-019-08077-z.
Asgharian H, Baniasadi E, Colpan CO. Energy, exergy and exergoeconomic analyses of a solar refrigeration cycle using nanofluid. Int J Exergy. 2019;30(1):63–85. https://doi.org/10.1504/IJEX.2019.101625.
Okonkwo EC, Wole-osho I, Kavaz D, Abid M, Al-ansari T. Thermodynamic evaluation and optimization of a flat plate collector operating with alumina and iron mono and hybrid nanofluids. Sustain Energy Technol Assess. 2020;37:100636. https://doi.org/10.1016/j.seta.2020.100636.
Dehaj MS, Mohiabadi MZ. Experimental study of water-based CuO nanofluid flow in heat pipe solar collector. J Therm Anal Calorim. 2019;137(6):2061–72. https://doi.org/10.1007/s10973-019-08046-6.
Shafieian A, Osman JJ, Khiadani M, Nosrati A. Enhancing heat pipe solar water heating systems performance using a novel variable mass flow rate technique and different solar working fluids. Sol Energy. 2019;186:191–203. https://doi.org/10.1016/j.solener.2019.05.016.
Karami M. Experimental investigation of first and second laws in a direct absorption solar collector using hybrid Fe3O4/SiO2 nanofluid. J Therm Anal Calorim. 2019;136(2):661–71. https://doi.org/10.1007/s10973-018-7624-x.
Alsaady M, Fu R, Yan Y, Liu Z, Wu S, Boukhanouf R. An experimental investigation on the effect of ferrofluids on the efficiency of novel parabolic trough solar collector under laminar flow conditions. Heat Transf Eng. 2019;40(9/10):753–61. https://doi.org/10.1080/01457632.2018.1442309.
Okonkwo EC, Abid M, Ratlamwala TAH, Abbasoglu S, Dagbasi M. Optimal analysis of entropy generation and heat transfer in parabolic trough collector using green-synthesized TiO2/water nanofluids. J Sol Energy Eng. 2019;141(3):031011.
Tayebi R, Akbarzadeh S, Valipour MS. Numerical investigation of efficiency enhancement in a direct absorption parabolic trough collector occupied by a porous medium and saturated by a nanofluid. Environ. Prog. Sustain. Energy. 2019;38(2):727–40. https://doi.org/10.1002/ep.13010.
Razmmand F, Mehdipour R, Mousavi SM. A numerical investigation on the effect of nanofluids on heat transfer of the solar parabolic trough collectors. Appl Therm Eng. 2019;152:624–33. https://doi.org/10.1016/j.applthermaleng.2019.02.118.
Al-Oran O, Lezsovits F, Aljawabrah A. Exergy and energy amelioration for parabolic trough collector using mono and hybrid nanofluids. J Therm Anal Calorim. 2020. https://doi.org/10.1007/s10973-020-09371-x.
Fathabadi H. Novel solar collector: evaluating the impact of nanoparticles added to the collector’s working fluid, heat transfer fluid temperature and flow rate. Renew Energy. 2019. https://doi.org/10.1016/j.renene.2019.10.008.
Kasaeian A, Daneshazarian R, Pourfayaz F, Babaei S, Sheikhpour M, Nakhjavani S. Evaluation of MWCNT/ethylene glycol nanofluid flow in a parabolic trough collector with glass-glass absorber tube. Int J Numer Methods Heat Fluid Flow. 2019. https://doi.org/10.1108/hff-11-2018-0693.
Hadi AI, Jamel MS. Effect of the design and environment parameters on the thermal efficiency and heat losses of a parabolic trough solar collector using nanofluid technology. Int J Mech Eng Technol. 2019;10(1):571–93.
Kumar D, Kumari S. Performance investigation of a nanofluid-based parabolic trough solar collector. In: Kumar M, Pandey R, Kumar V, editors. Advances in interdisciplinary engineering, vol. 1., Lecture Notes in Mechanical EngineeringSingapore: Springer; 2019.
Islam MK, Hasanuzzaman M, Rahim NA, Nahar A. Effect of nanofluid properties and mass flow rate on heat transfer of on heat transfer of parabolic trough concentrating solar system. J Nav Archit Mar Eng. 2019;16(1):33–44. https://doi.org/10.3329/jname.v16i1.30548.
Norouzi AM, Siavashi M, Oskouei MHK. Efficiency enhancement of the parabolic trough solar collector using the rotating absorber tube and nanoparticles. Renew Energy. 2020;145:569–84. https://doi.org/10.1016/j.renene.2019.06.027.
Bozorg MV, Doranehgard MH, Hong K, **ong Q. CFD study of heat transfer and fluid flow in a parabolic trough solar receiver with internal annular porous structure and synthetic oil–Al2O3 nanofluid. Renew Energy. 2020;145:2598–614. https://doi.org/10.1016/j.renene.2019.08.042.
Quezada-García S, Sánchez-Mora H, Polo-Labarrios MA, Cázares-Ramírez RI. Modeling and simulation to determine the thermal efficiency of a parabolic solar trough collector system. Case Stud Therm Eng. 2019;16:100523. https://doi.org/10.1016/j.csite.2019.100523.
Campos CS, Torres JPN, Fernandes JFP. Effects of the heat transfer fluid selection on the efficiency of a hybrid concentrated photovoltaic and thermal collector. Energies. 2019;12(9):1–12. https://doi.org/10.3390/en12091814.
Gangadevi R, Vinayagam BK. Experimental determination of thermal conductivity and viscosity of different nanofluids and its effect on a hybrid solar collector. J Therm Anal Calorim. 2019;136(1):199–209. https://doi.org/10.1007/s10973-018-7840-4.
Ahmed OK, Bawa SM. The combined effect of nanofluid and reflective mirrors on the performance of PV/Thermal solar collector. Therm Sci. 2019;23(2):573–87. https://doi.org/10.2298/TSCI171203092A.
Qi C, Luo T, Liu M, Fan F, Yan Y. Experimental study on the flow and heat transfer characteristics of nanofluids in double-tube heat exchangers based on thermal efficiency assessment. Energy Convers Manag. 2019;197:111877. https://doi.org/10.1016/j.enconman.2019.111877.
Moradi A, Toghraie D, Isfahani AHM, Hosseinian A. An experimental study on MWCNT–water nanofluids flow and heat transfer in double-pipe heat exchanger using porous media. J Therm Anal Calorim. 2019;137(5):1797–807. https://doi.org/10.1007/s10973-019-08076-0.
Mohankumar T, Rajan K, Sivakumar K, Gopal V. Experimental analysis of heat transfer characteristics of heat exchanger using nano fluids. IOP Conf Ser Mater Sci Eng. 2019;574(1):45–69. https://doi.org/10.1088/1757-899x/574/1/012011.
Mehta KS, Kundan L, Mallick SS. A study on heat transfer and pressure drop in a turbulent flow regime of thermally insulated and conducting nanofluids. J Nanofluids. 2019;8(3):490–9. https://doi.org/10.1166/jon.2019.1602.
Zheng M, Han D, Asif F, Si Z. Effect of Al2O3/water nanofluid on heat transfer of turbulent flow in the inner pipe of a double-pipe heat exchanger. Heat Mass Transf Stoffuebertragung. 2019. https://doi.org/10.1007/s00231-019-02774-z.
Returi MC, Konijeti R, Dasore A. Heat transfer enhancement using hybrid nanofluids in spiral plate heat exchangers. Heat Transf Res. 2019;48(7):3128–43. https://doi.org/10.1002/htj.21534.
Kayabaşı U, Kakaç S, Aradag S, Pramuanjaroenkij A. Experimental investigation of thermal and hydraulic performance of a plate heat exchanger using nanofluids. J Eng Phys Thermophys. 2019;92(3):783–96. https://doi.org/10.1007/s10891-019-01987-7.
Variyenli Hİ. Experimental and numerical investigation of heat transfer enhancement in a plate heat exchanger using a fly ash nanofluid. Heat Transf Res. 2019;50(15):1477–94. https://doi.org/10.1615/HeatTransRes.2019029136.
Meisam A, Ahmad A, Hamed M. Experimental investigation of metal oxide nanofluids in a plate heat exchanger. J Thermophys Heat Transf. 2019;33(4):994–1005. https://doi.org/10.2514/1.T5581.
Teng TP, Hsiao TC, Chung CC. Characteristics of carbon-based nanofluids and their application in a brazed plate heat exchanger under laminar flow. Appl Therm Eng. 2019;146:160–8. https://doi.org/10.1016/j.applthermaleng.2018.09.125.
Hosseini SM, Safaei MR, Estellé P, Jafarnia SH. Heat transfer of water-based carbon nanotube nanofluids in the shell and tube cooling heat exchangers of the gasoline product of the residue fluid catalytic cracking unit. J Therm Anal Calorim. 2019;56:2019. https://doi.org/10.1007/s10973-019-08813-5.
Naik BAK, Vinod AV. Energy savings and effectiveness in a compact heat exchanger employing non-newtonian nanofluids. J Nanofluids. 2019;8(7):1535–43. https://doi.org/10.1166/jon.2019.1700.
Permanasari AA, Kuncara BS, Puspitasari P, Sukarni S, Ginta TL, Irdianto W. Convective heat transfer characteristics of TiO2-EG nanofluid as coolant fluid in heat exchanger. AIP Conf Proc. 2019;2120:56. https://doi.org/10.1063/1.5115691.
Said Z, Rahman SMA, El Assad MH, Alami AH. Heat transfer enhancement and life cycle analysis of a Shell-and-Tube Heat Exchanger using stable CuO/water nanofluid. Sustain Energy Technol Assess. 2019;31:306–17. https://doi.org/10.1016/j.seta.2018.12.020.
Ullah MR, Ishtiaq TM, Mamun MAH. Heat transfer enhancement in shell and tube heat exchanger by using Al2O3/water and TiO2/water nanofluid. AIP Conf Proc. 2019;2121:2019. https://doi.org/10.1063/1.5115925.
Khanlari A, Sözen A, Variyenli HI, Gürü M. Comparison between heat transfer characteristics of TiO2/deionized water and kaolin/deionized water nanofluids in the plate heat exchanger. Heat Transf Res. 2019;50(5):435–50. https://doi.org/10.1615/heattransres.2018026288.
Ali AYM, El-Shazly AH, El-Kady MF, Elqady HI, El-Marghany MR. Effect of using MgO-oil nanofluid on the performance of a counter-flow double pipe heat exchanger. Key Eng Mater. 2019;801:193–8. https://doi.org/10.4028/www.scientific.net/KEM.801.193.
Kumar V, Pandya N, Pandya B, Joshi A. Synthesis of metal-based nanofluids and their thermo-hydraulic performance in compact heat exchanger with multi-louvered fins working under laminar conditions. J Therm Anal Calorim. 2019;135(4):2221–35. https://doi.org/10.1007/s10973-018-7304-x.
Suroso B, Kamal S, Kristiawan B, Irawansyah H, Wibowo BS, Yani M. Convective heat transfer of nanofluids TiO2/Thermo Oil XT 32 in concentric tube heat exchanger. IOP Conf Ser Mater Sci Eng. 2019. https://doi.org/10.1088/1757-899x/674/1/012063.
Subramanian R, Kumar AS, Vinayagar K, Muthusamy C. Experimental analyses on heat transfer performance of TiO2–water nanofluid in double-pipe counter-flow heat exchanger for various flow regimes. J Therm Anal Calorim. 2019. https://doi.org/10.1007/s10973-019-08887-1.
Monfared M, Shahsavar A, Bahrebar MR. Second law analysis of turbulent convection flow of boehmite alumina nanofluid inside a double-pipe heat exchanger considering various shapes for nanoparticle. J Therm Anal Calorim. 2019;135(2):1521–32. https://doi.org/10.1007/s10973-018-7708-7.
Shirzad M, Ajarostaghi SSM, Delavar MA, Sedighi K. Improve the thermal performance of the pillow plate heat exchanger by using nanofluid: numerical simulation. Adv Powder Technol. 2019;30(7):1356–65. https://doi.org/10.1016/j.apt.2019.04.011.
Kumar SD, Purushothaman K. Enhancement of thermal conductivity in a plate heat exchanger by using nanoparticles CNT, Al2O3, surfactant with de-ionised water as a coolant. Int J Ambient Energy. 2019. https://doi.org/10.1080/01430750.2018.1562979.
Koshta NR, Bhanvase BA, Chawhan SS, Barai DP, Sonawane SH. Investigation on the thermal conductivity and convective heat transfer enhancement in helical coiled heat exchanger using ultrasonically prepared rGO–TiO2 nanocomposite-based nanofluids. Indian Chem Eng. 2019. https://doi.org/10.1080/00194506.2019.1658545.
Solangi KH, Sharif S, Nizamani B. Effect of tube material on convective heat transfer of various nanofluids. J Therm Anal Calorim. 2019. https://doi.org/10.1007/s10973-019-08835-z.
Nikkhah V, Nakhjavani S. Thermal performance of a micro heat exchanger (MHE) working with zirconia-based nanofluids for industrial cooling. Int J Ind Chem. 2019;10(2):193–204. https://doi.org/10.1007/s40090-019-0183-6.
Sözen A, Khanları A, Çiftçi E. Heat transfer enhancement of plate heat exchanger utilizing kaolin-including working fluid. Proc Inst Mech Eng Part A J Power Energy. 2019;233(5):626–34. https://doi.org/10.1177/0957650919832445.
Gustavo J, Fnu A, Valentin S, Mosfequr R. Erosion-corrosion wear of heat-exchanger materials by water/ethylene-glycol/alumina nanofluids. Int J Surf Eng Interdiscip Mater Sci. 2018;6(2):1–22.
Ramalingam S, Dhairiyasamy R, Govindasamy M. Consequence of nanoparticle physiognomies on heat transfer characteristics of heat exchanger. Energy Sources Part A Recover Util Environ Eff. 2019. https://doi.org/10.1080/15567036.2019.1670759.
Tian Z, et al. Turbulent flows in a spiral double-pipe heat exchanger: optimal performance conditions using an enhanced genetic algorithm. Int J Numer Methods Heat Fluid Flow. 2019. https://doi.org/10.1108/hff-04-2019-0287.
Shahsavar A, Rahimi Z, Salehipour H. Nanoparticle shape effects on thermal-hydraulic performance of boehmite alumina nanofluid in a horizontal double-pipe minichannel heat exchanger. Heat Mass Transf Stoffuebertragung. 2019;55(6):1741–51. https://doi.org/10.1007/s00231-018-02558-x.
Khanlari A, Sözen A, Variyenli Hİ. Simulation and experimental analysis of heat transfer characteristics in the plate type heat exchangers using TiO2/water nanofluid. Int J Numer Methods Heat Fluid Flow. 2019;29(4):1343–62. https://doi.org/10.1108/HFF-05-2018-0191.
Osman S, Sharifpur M, Meyer JP. Experimental investigation of convection heat transfer in the transition flow regime of aluminium oxide-water nanofluids in a rectangular channel. Int J Heat Mass Transf. 2019;133:895–902. https://doi.org/10.1016/j.ijheatmasstransfer.2018.12.169.
Bhattad A, Sarkar J, Ghosh P. Experimentation on effect of particle ratio on hydrothermal performance of plate heat exchanger using hybrid nanofluid. Appl Therm Eng. 2019;162:114309. https://doi.org/10.1016/j.applthermaleng.2019.114309.
Bhattad A, Sarkar J, Ghosh P. Hydrothermal performance of different alumina hybrid nanofluid types in plate heat exchanger: experimental study. J Therm Anal Calorim. 2019. https://doi.org/10.1007/s10973-019-08682-y.
Ozdemir MB, Ergun ME. Experimental and numerical investigations of thermal performance of Al2O3/water nanofluid for a combi boiler with double heat exchangers. Int J Numer Methods Heat Fluid Flow. 2019;29(4):1300–21. https://doi.org/10.1108/HFF-05-2018-0189.
Talari VK, Thamida SK, Sastry RC. Determination of optimum concentration of nanofluid for process intensification of heat transfer using corrugated plate type heat exchanger. Chem Prod Process Model. 2019;14(1):2019. https://doi.org/10.1515/cppm-2018-0002.
Arulprakasajothi M, Raja ND, Beemkumar N, Elangovan K. Experimental study on Al2O3/H2O nanofluid with conical sectional insert in concentric tube heat exchanger. Energy Sources Part A Recover Util Environ Eff. 2019. https://doi.org/10.1080/15567036.2019.1649753.
Mansoury D, Doshmanziari FI, Rezaie S, Rashidi MM. Effect of Al2O3/water nanofluid on performance of parallel flow heat exchangers: an experimental approach. J Therm Anal Calorim. 2019;135(1):625–43. https://doi.org/10.1007/s10973-018-7286-8.
Kong R, Deethayat T, Asanakham A, Kiatsiriroat T. Thermal characteristics of helical coiled heat exchanger with graphene-deionized water on waste heat recovery of combustion stack gas. Chiang Mai Univ J Nat Sci. 2019;18(1):50–67. https://doi.org/10.12982/CMUJNS.2019.0005.
Liu WI, Al-Rashed AAAA, Alsagri AS, Mahmoudi B, Shahsavar A, Afrand M. Laminar forced convection performance of non-Newtonian water-CNT/Fe3O4 nano-fluid inside a minichannel hairpin heat exchanger: effect of inlet temperature. Powder Technol. 2019;354:247–58. https://doi.org/10.1016/j.powtec.2019.05.079.
Toghraie D, Hekmatifar M, Jolfaei NA. Investigation of heat transfer and fluid flow behaviors of CuO/(60:40)% ethylene glycol and water nanofluid through a serpentine milichannel heat exchanger; 2019.
Soman DP, Karthika S, Kalaichelvi P, Radhakrishnan TK. Experimental study of turbulent forced convection heat transfer and friction factor in dimpled plate heat exchanger. Appl Therm Eng. 2019;162:2019. https://doi.org/10.1016/j.applthermaleng.2019.114254.
Mansoury D, Doshmanziari FI, Kiani A, Chamkha AJ, Sharifpur M. Heat transfer and flow characteristics of Al2O3/water nanofluid in various heat exchangers: experiments on counter flow. Heat Transf Eng. 2019. https://doi.org/10.1080/01457632.2018.1528051.
Anvari AR, Javaherdeh K, Emami-Meibodi M. Investigation of heat transfer and pressure drop of non-newtonian nanofluid performance through micro channels heat exchanger (MCHE) in cross-flow configuration. J Nanofluids. 2019;8(3):631–9. https://doi.org/10.1166/jon.2019.1600.
Anitha S, Thomas T, Parthiban V, Pichumani M. What dominates heat transfer performance of hybrid nanofluid in single pass shell and tube heat exchanger? Adv Powder Technol. 2019;30(12):3107–17. https://doi.org/10.1016/j.apt.2019.09.018.
Sundar LS, Kumar NTR, Addis BM, Bhramara P, Singh MK, Sousa ACM. Heat transfer and effectiveness experimentally-based analysis of wire coil with core-rod inserted in Fe3O4/water nanofluid flow in a double pipe U-bend heat exchanger. Int J Heat Mass Transf. 2019;134:405–19. https://doi.org/10.1016/j.ijheatmasstransfer.2019.01.041.
Attalla M, Maghrabie HM. An experimental study on heat transfer and fluid flow of rough plate heat exchanger using Al2O3/water nanofluid. Exp Heat Transf. 2019. https://doi.org/10.1080/08916152.2019.1625469.
Mikhailenko SA, Sheremet MA, Pop I. Convective heat transfer in a rotating nanofluid cavity with sinusoidal temperature boundary condition. J Therm Anal Calorim. 2019;137(3):799–809. https://doi.org/10.1007/s10973-018-7984-2.
Bondarenko DS, Sheremet MA, Oztop HF, Ali ME. Impacts of moving wall and heat-generating element on heat transfer and entropy generation of Al2O3/H2O nanofluid. J Therm Anal Calorim. 2019;136(2):673–86. https://doi.org/10.1007/s10973-018-7715-8.
Sheremet MA, Pop I. Marangoni natural convection in a cubical cavity filled with a nanofluid: buongiorno’s nanofluid model. J Therm Anal Calorim. 2019;135(1):357–69. https://doi.org/10.1007/s10973-018-7069-2.
Bondarenko DS, Sheremet MA, Oztop HF, Ali ME. Natural convection of Al2O3/H2O nanofluid in a cavity with a heat-generating element. Heatline visualization. Int J Heat Mass Transf. 2019;130:564–74. https://doi.org/10.1016/j.ijheatmasstransfer.2018.10.091.
Mikhailenko SA, Sheremet MA, Oztop HF, Abu-Hamdeh N. Thermal convection in Al2O3–water nanoliquid rotating chamber with a local isothermal heater. Int J Mech Sci. 2019;156:137–45. https://doi.org/10.1016/j.ijmecsci.2019.03.037.
Dogonchi AS, Sheremet MA, Ganji DD, Pop I. Free convection of copper–water nanofluid in a porous gap between hot rectangular cylinder and cold circular cylinder under the effect of inclined magnetic field. J Therm Anal Calorim. 2019;135(2):1171–84. https://doi.org/10.1007/s10973-018-7396-3.
Bondarenko DS, Sheremet MA, Oztop HF, Abu-Hamdeh N. Mixed convection heat transfer of a nanofluid in a lid-driven enclosure with two adherent porous blocks. J Therm Anal Calorim. 2019;135(2):1095–105. https://doi.org/10.1007/s10973-018-7455-9.
Sheremet MA, Pop I, Baytas AC. Non-equilibrium natural convection in a differentially-heated nanofluid cavity partially filled with a porous medium. Int J Numer Methods Heat Fluid Flow. 2019;29(8):2524–44. https://doi.org/10.1108/HFF-08-2018-0433.
Buonomo B, Manca O, Bondareva NS, Sheremet MA. Thermal and fluid dynamic behaviors of confined slot jets im**ing on an isothermal moving surface with nanofluids. Energies. 2019. https://doi.org/10.3390/en12112074.
Ma Y, Mohebbi R, Rashidi MM, Yang Z, Sheremet MA. Numerical study of MHD nanofluid natural convection in a baffled U-shaped enclosure. Int J Heat Mass Transf. 2019;130:123–34. https://doi.org/10.1016/j.ijheatmasstransfer.2018.10.072.
Vishnuprasad S, Haribabu K, Perarasu V. Experimental study on the convective heat transfer performance and pressure drop of functionalized graphene nanofluids in electronics cooling system. Heat Mass Transf. 2019;55(8):2221–34. https://doi.org/10.3795/KSME-B.2019.43.9.661.
Joy RC, Rajan AA, Solomon AB, Ramachandran K, Pillai BC. Experimental investigation on the critical heat flux of Cu-water, Al-water nanofluids for precise cooling of electronic systems. IOP Conf Ser Mater Sci Eng. 2019;561:1. https://doi.org/10.1088/1757-899x/561/1/012036.
Zing C, Mahjoob S, Vafai K. Analysis of porous filled heat exchangers for electronic cooling. Int J Heat Mass Transf. 2019;133:268–76. https://doi.org/10.1016/j.ijheatmasstransfer.2018.12.067.
Bezaatpour M, Goharkhah M. A novel heat sink design for simultaneous heat transfer enhancement and pressure drop reduction utilizing porous fins and magnetite ferrofluid. Int J Numer Methods Heat Fluid Flow. 2019;29(9):3128–47. https://doi.org/10.1108/HFF-12-2018-0810.
Al-Rashed A, Shahsavar A, Entezari S, Moghimi MA, Adio SA, Nguyen TK. Numerical investigation of non-Newtonian water-CMC/CuO nanofluid flow in an offset strip-fin microchannel heat sink: thermal performance and thermodynamic considerations. Appl Therm Eng. 2019;155:247–58. https://doi.org/10.1016/j.applthermaleng.2019.04.009.
Qiu L, et al. Coating-boosted interfacial thermal transport for carbon nanotube array nano-thermal interface materials. Carbon N. Y. 2019;145:725–33. https://doi.org/10.1016/j.carbon.2019.01.085.
Hong F, Cheng P. Three dimensional numerical analyses and optimization of offset strip-fin microchannel heat sinks. Int Commun Heat Mass Transf. 2009;36:651–6. https://doi.org/10.1016/j.icheatmasstransfer.2009.02.015.
Saghir MZ, Welsford C, Thanapathy P, Bayomy AM, Delisle C. Experimental measurements and numerical computation of nano heat transfer enhancement inside a porous material. J Therm Sci Eng Appl. 2020;12:1. https://doi.org/10.1115/1.4041936.
Ali M, Shoukat AA, Tariq HA, Anwar M, Ali H. Header design optimization of mini-channel heat sinks using CuO–H2O and Al2O3–H2O nanofluids for thermal management. Arab J Sci Eng. 2019;44(12):10327–38. https://doi.org/10.1007/s13369-019-04022-2.
Hadavand M, Yousefzadeh S, Akbari OA, Pourfattah F, Nguyen HM, Asadi A. A numerical investigation on the effects of mixed convection of Ag-water nanofluid inside a sim-circular lid-driven cavity on the temperature of an electronic silicon chip. Appl Therm Eng. 2019;162:114298. https://doi.org/10.1016/j.applthermaleng.2019.114298.
Bahiraei M, Heshmatian S, Goodarzi M, Moayedi H. CFD analysis of employing a novel ecofriendly nanofluid in a miniature pin fin heat sink for cooling of electronic components: effect of different configurations. Adv Powder Technol. 2019;30:2503–16. https://doi.org/10.1016/j.apt.2019.07.029.
Fadhil AM, Khalil WH, Al-damook A. The hydraulic-thermal performance of miniature compact heat sinks using SiO2-water nanofluids. Heat Transf Asian Res. 2019;48(7):3101–14. https://doi.org/10.1002/htj.21532.
Riehl RR. Thermal enhancement using nanofluids on high heat dissipation electronic components. J Nanofluids. 2019;8(1):30–40. https://doi.org/10.1166/jon.2019.1563.
Ma H, Duan Z, Su L, Ning X, Bai J, Lv X. Fluid flow and entropy generation analysis of Al2O3–water nanofluid in microchannel plate fin heat sinks. Entropy. 2019;21(8):739.
Narendran G, Gnanasekaran N, Perumal DA. Thermodynamic irreversibility and conjugate effects of integrated microchannel cooling device using TiO2 nanofluid. Heat Mass Transf Stoffuebertragung. 2019. https://doi.org/10.1007/s00231-019-02704-z.
Goodarzi M, Tlili I, Tian Z, Safaei MR. Efficiency assessment of using graphene nanoplatelets-silver/water nanofluids in microchannel heat sinks with different cross-sections for electronics cooling. Int J Numer Methods Heat Fluid Flow. 2019. https://doi.org/10.1108/HFF-12-2018-0730.
Alsarraf J, Shahsavar A, Khaki M, Ranjbarzadeh R, Karimipour A, Afrand M. Numerical investigation on the effect of four constant temperature pipes on natural cooling of electronic heat sink by nanofluids: a multifunctional optimization. Adv Powder Technol. 2019. https://doi.org/10.1016/j.apt.2019.10.035.
Adham AM. Ammonia base nanofluid as a coolant for electronic chips. Int J Mech Prod Eng Res Dev. 2019;9(3):569–80. https://doi.org/10.24247/ijmperdjun201960.
Baïri A, Bauzin JG, Martín-Garín A, Alilat N, Millán-García JA. Natural convective cooling of electronics contained in tilted hemispherical enclosure filled with a porous medium saturated by water-copper nanofluid. Int J Numer Methods Heat Fluid Flow. 2019;29(1):280–93. https://doi.org/10.1108/HFF-01-2018-0036.
Baïri A, Laraqi N. Experimental quantification of natural convective heat transfer within annulus space filled with a H2O-Cu nanofluid saturated porous medium. Application to electronics cooling. Exp Heat Transf. 2019;32(4):364–75. https://doi.org/10.1080/08916152.2018.1526230.
Yang L, Huang JN, Mao M, Ji W. Numerical assessment of Ag-water nano-fluid flow in two new microchannel heatsinks: thermal performance and thermodynamic considerations. Int Commun Heat Mass Transf. 2020;110:104415. https://doi.org/10.1016/j.icheatmasstransfer.2019.104415.
Yang L, Du K, Zhang Z. Heat transfer and flow optimization of a novel sinusoidal minitube filled with non-Newtonian SiC/EG-water nanofluids. Int J Mech Sci. 2020;168:105310. https://doi.org/10.1016/j.ijmecsci.2019.105310.
Elsaid AM. Experimental study on the heat transfer performance and friction factor characteristics of CO3O4 and Al2O3 based H2O/(CH2OH)2 nanofluids in a vehicle engine radiator. Int Commun Heat Mass Transf. 2019;108:104263. https://doi.org/10.1016/j.icheatmasstransfer.2019.05.009.
Naiman I, Ramasamy D, Kadirgama K. Experimental and one dimensional investigation on nanocellulose and aluminium oxide hybrid nanofluid as a new coolant for radiator. IOP Conf Ser Mater Sci Eng. 2019;469:1. https://doi.org/10.1088/1757-899x/469/1/012096.
Al Rafi A, Haque R, Sikandar F, Chowdhury NA. Experimental analysis of heat transfer with CuO, Al2O3/water-ethylene glycol nanofluids in automobile radiator. In: AIP Conference Proceedings; 2019, vol. 2121, https://doi.org/10.1063/1.5115878.
Kumar V, Sahoo RR. Exergy and energy analysis of a wavy fin radiator with variously shaped nanofluids as coolants. Heat Transf Asian Res. 2019;48(6):2174–92. https://doi.org/10.1002/htj.21478.
Contreras EMC, Oliveira GA, Filho EPB. Experimental analysis of the thermohydraulic performance of graphene and silver nanofluids in automotive cooling systems. Int J Heat Mass Transf. 2019;132:375–87. https://doi.org/10.1016/j.ijheatmasstransfer.2018.12.014.
Said Z, et al. Enhancing the performance of automotive radiators using nanofluids. Renew Sustain Energy Rev. 2019;112:183–94. https://doi.org/10.1016/j.rser.2019.05.052.
Mahay N, Yadav RK. An experimental investigation into heat transfer characteristics of aqua based Cu nanofluid for automobile radiator. J Phys Conf Ser. 2019. https://doi.org/10.1088/1742-6596/1240/1/012043.
Soylu SK, Atmaca İ, Asiltürk M, Doğan A. Improving heat transfer performance of an automobile radiator using Cu and Ag doped TiO2 based nanofluids. Appl Therm Eng. 2019;157:113743. https://doi.org/10.1016/j.applthermaleng.2019.113743.
Arunkumar T, Anish M, Jayaprabakar J, Beemkumar N. Enhancing heat transfer rate in a car radiator by using Al2O3 nanofluid as a coolant. Int J Ambient Energy. 2019;40(4):367–73. https://doi.org/10.1080/01430750.2017.1392356.
Palaniappan B, Ramasamy V. Thermodynamic analysis of fly ash nanofluid for automobile (heavy vehicle) radiators. J Therm Anal Calorim. 2019;136(1):223–33. https://doi.org/10.1007/s10973-018-7844-0.
Jadar R, Shashishekar KS, Manohara SR. Performance evaluation of Al-MWCNT based automobile radiator. Mater Today Proc. 2019;9:380–8. https://doi.org/10.1016/j.matpr.2019.02.167.
Toh LKL, Ting TW. Thermal performance of automotive radiator with graphene nanoplatelets suspension. AIP Conf Proc. 2019;2059:020012. https://doi.org/10.1063/1.5085955.
Maisuria MB, Sonar DM, Rathod MK, Bhatt MK. Experimental and analytical investigation on an automobile radiator with CuO/EG-water based nanofluid as coolant. Heat Transf Asian Res. 2019;48(6):2596–612. https://doi.org/10.1002/htj.21516.
Zhou XR, Wang Y, Zheng K, Huang H. Comparison of heat transfer performance of ZnO-PG, α-Al2O3-PG, and γ-Al2O3-PG nanofluids in car radiator. Nanomater Nanotechnol. 2019. https://doi.org/10.1177/1847980419876465.
Akash AR, Abraham S, Pattamatta A, Das SK. Experimental assessment of the thermo-hydraulic performance of automobile radiator with metallic and nonmetallic nanofluids. Heat Transf Eng. 2019;41(3):235–51. https://doi.org/10.1080/01457632.2018.1528055.
Akash A, Pattamatta A, Das S. Experimental study of the thermohydraulic performance of water/ethylene glycol–based graphite nanocoolant in vehicle radiators. J Enhanc Heat Transf. 2019;26(4):345–63.
Bondareva NS, Buonomo B, Manca O, Sheremet MA. Heat transfer performance of the finned nano-enhanced phase change material system under the inclination influence. Int J Heat Mass Transf. 2019;135:1063–72. https://doi.org/10.1016/j.ijheatmasstransfer.2019.02.045.
Navarrete N, Mondragón R, Wen D, Navarro ME, Ding Y, Juliá JE. Thermal energy storage of molten salt –based nanofluid containing nano-encapsulated metal alloy phase change materials. Energy. 2019;167:912–9. https://doi.org/10.1016/j.energy.2018.11.037.
Martín M, Villalba A, Fernández AI, Barreneche C. Development of new nano-enhanced phase change materials (NEPCM) to improve energy efficiency in buildings: Lab-scale characterization. Energy Build. 2019;192:75–83. https://doi.org/10.1016/j.enbuild.2019.03.029.
Ding M, Liu C, Rao Z. Experimental investigation on heat transfer characteristic of TiO2-H2O nanofluid in microchannel for thermal energy storage. Appl Therm Eng. 2019;160:114024. https://doi.org/10.1016/j.applthermaleng.2019.114024.
Harikrishnan S, Devaraju A, Kumar GR, Kalaiselvam S. Improved thermal energy storage behavior of a novel nanofluid as phase change material (PCM). Mater Today Proc. 2019;9:410–21. https://doi.org/10.1016/j.matpr.2019.02.170.
Cabeza LF, Castell A, Barreneche C, De Gracia A, Fernández AI. Materials used as PCM in thermal energy storage in buildings: a review. Renew Sustain Energy Rev. 2011;15:1675–95. https://doi.org/10.1016/j.rser.2010.11.018.
Wang Q, Tang S, Tian S, Wei X, Peng T. Molecular simulations of adsorption and thermal energy storage of mixed R1234Ze/UiO-66 nanoparticle nanofluid. J Nanomater. 2019. https://doi.org/10.1155/2019/5154173.
Zhou Y, Li Q, Wang Q. Energy storage analysis of UIO-66 and water mixed nanofluids: an experimental and theoretical study. Energies. 2019;12(13):1–9. https://doi.org/10.3390/en12132521.
Hassan MAM, Abdel-Hameed HM, Mahmoud OE. Experimental investigation of the effect of nanofluid on thermal energy storage system using clathrate. J Energy Resour Technol Trans ASME. 2019;141(4):1–8. https://doi.org/10.1115/1.4042004.
Hu Y, He Y, Zhang Z, Wen D. Enhanced heat capacity of binary nitrate eutectic salt-silica nanofluid for solar energy storage. Sol Energy Mater Sol Cells. 2019;192:94–102. https://doi.org/10.1016/j.solmat.2018.12.019.
Sheikholeslami M, Jafaryar M, Shafee A, Li Z. Hydrothermal and second law behavior for charging of NEPCM in a two dimensional thermal storage unit. Chin J Phys. 2019;58:244–52. https://doi.org/10.1016/j.cjph.2019.02.001.
Chen M, He Y, Ye Q, Zhang Z, Hu Y. Solar thermal conversion and thermal energy storage of CuO/Paraffin phase change composites. Int J Heat Mass Transf. 2019;130:1133–40. https://doi.org/10.1016/j.ijheatmasstransfer.2018.11.026.
Prasad AR, Kumar SKD, Banu TV, Vignesh T, Aatthisugan I. Synthesis and thermal energy storage analysis of copper oxide nano fluid for heat transfer applications. Int J Innov Technol Explor Eng. 2019;8(11):3616–9. https://doi.org/10.35940/ijitee.k2472.0981119.
Nithiyanantham U, Grosu Y, González-Fernández L, Zaki A, Igartua JM, Faik A. Corrosion aspects of molten nitrate salt-based nanofluids for thermal energy storage applications. Sol Energy. 2019;189:219–27. https://doi.org/10.1016/j.solener.2019.07.050.
Farsani RY, Raisi A, Mahmoudi A. Successive melting and solidification of paraffin–alumina nanomaterial in a cavity as a latent heat thermal energy storage. J Braz Soc Mech Sci Eng. 2019. https://doi.org/10.1007/s40430-019-1859-8.
Qiao G, She X, Zhang T, Cong L, Chen YC, Ding Y. Mechanism of specific heat capacity enhancement of molten salts based nanofluids for thermal energy storage—a molecular study. In: 2019 Offshore Energy and Storage Summit, OSES 2019; 2019. pp. 1–7, https://doi.org/10.1109/OSES.2019.8867356.
Ji J, et al. Fabrication and characterization of phase change nanofluid with high thermophysical properties for thermal energy storage. J Mol Liq. 2019;284:23–8. https://doi.org/10.1016/j.molliq.2019.03.116.
Hadi A, Rashid FL, Hussein HQ, Hashim A. Novel of water with (CeO2-WC) and (SiC-WC) nanoparticles systems for energy storage and release applications. IOP Conf Ser Mater Sci Eng. 2019;518:3. https://doi.org/10.1088/1757-899x/518/3/032059.
Sheikholeslami M, Shehzad SA, Li Z, Shafee A, Abbasi FM. Time dependent conduction heat transfer during solidification in a storage system using nanoparticles. Microsyst Technol. 2019;25(6):2153–69. https://doi.org/10.1007/s00542-018-4050-8.
Rahman S, Issa S, Said Z, El Assad MH, Zadeh R, Barani Y. Performance enhancement of a solar powered air conditioning system using passive techniques and SWCNT/R-407c nano refrigerant. Case Stud Therm Eng. 2019;16:100565. https://doi.org/10.1016/j.csite.2019.100565.
Jiang W, Li S, Yang L, Du K. Experimental investigation on performance of ammonia absorption refrigeration system with TiO2 nanofluid. Int J Refrig. 2019;98:80–8. https://doi.org/10.1016/j.ijrefrig.2018.09.032.
Jeyakumar N, Uthranarayan C, Narayanasamy B. Energy conservation in the refrigeration system through improvement of Coefficient of Performance and power consumption reduction using Nanofluids. Int J Ambient Energy. 2019. https://doi.org/10.1080/01430750.2019.1687333.
Ahmed MS, Elsaid AM. Effect of hybrid and single nanofluids on the performance characteristics of chilled water air conditioning system. Appl Therm Eng. 2019;163:114398. https://doi.org/10.1016/j.applthermaleng.2019.114398.
Nourafkan E, Asachi M, ** H, Wen D, Ahmed W. Stability and photo-thermal conversion performance of binary nanofluids for solar absorption refrigeration systems. Renew Energy. 2019;140:24–273. https://doi.org/10.1016/j.renene.2019.01.081.
Esfe MH, Dalir R, Bakhtiari R, Afrand M. Simultaneous effects of multi-walled carbon nanotubes and copper oxide nanoparticles on the rheological behavior of cooling oil: application for refrigeration systems. Int J Refrig. 2019;104:123–33. https://doi.org/10.1016/j.ijrefrig.2018.11.036.
Aprea C, Greco A, Maiorino A, Masselli C. Enhancing the heat transfer in an active barocaloric cooling system using ethylene-glycol based nanofluids as secondary medium. Energies. 2019;12:15. https://doi.org/10.3390/en12152902.
Safaei MR, Ranjbarzadeh R, Hajizadeh A, Bahiraei M, Afrand M, Karimipour A. Effects of cobalt ferrite coated with silica nanocomposite on the thermal conductivity of an antifreeze: new nanofluid for refrigeration condensers. Int J Refrig. 2019;102:86–95. https://doi.org/10.1016/j.ijrefrig.2018.12.007.
Mohammed HI, Giddings D, Walker GS. Experimental investigation of nanoparticles concentration, boiler temperature and flow rate on flow boiling of zinc bromide and acetone solution in a rectangular duct. Int J Heat Mass Transf. 2019;130:710–21. https://doi.org/10.1016/j.ijheatmasstransfer.2018.10.115.
Pourfayaz F, Imani M, Mehrpooya M, Shirmohammadi R. Process development and exergy analysis of a novel hybrid fuel cell-absorption refrigeration system utilizing nanofluid as the absorbent liquid. Int J Refrig. 2019;97:31–41. https://doi.org/10.1016/j.ijrefrig.2018.09.011.
Bondre D, Joshi A, Shinde T, Deshmukh A, Dhanawade K. Experimental performance and analysis of domestic refrigeration system using nano-refrigerants. In: Proceedings of International Conference on Intelligent Manufacturing and Automation. Lecture Notes in Mechanical Engineering; 2019. https://doi.org/10.1007/978-981-13-2490-1_35.
Modi N, Pandya B, Patel J. Comparative analysis of a solar-driven novel salt-based absorption chiller with the implementation of nanoparticles. Int J Energy Res. 2019;43(4):1563–77. https://doi.org/10.1002/er.4405.
Adelekan DS, Ohunakin OS, Gill J, Atiba OE, Okokpujie IP, Atayero AA. Experimental investigation of a vapour compression refrigeration system with 15 nm TiO2-R600a nano-refrigerant as the working fluid. In: Procedia Manufacturing; 2019. pp. 1222–1227, https://doi.org/10.1016/j.promfg.2019.06.079.
Nazari S, Safarzadeh H, Bahiraei M. Experimental and analytical investigations of productivity, energy and exergy efficiency of a single slope solar still enhanced with thermoelectric channel and nanofluid. Renew Energy. 2019;135:729–44. https://doi.org/10.1016/j.renene.2018.12.059.
Nazari S, Safarzadeh H, Bahiraei M. Performance improvement of a single slope solar still by employing thermoelectric cooling channel and copper oxide nanofluid: an experimental study. J Clean Prod. 2019;208:1041–52. https://doi.org/10.1016/j.jclepro.2018.10.194.
Rafiq M, Chengrong L, Lv Y. Effect of Al2O3 nanorods on dielectric strength of aged transformer oil/paper insulation system. J Mol Liq. 2019;284:700–8. https://doi.org/10.1016/j.molliq.2019.04.041.
Pak BC, Cho YI. Hydrodynamic and heat transfer study of dispersed fluids with submicron metallic oxide particles. Exp Heat Transf. 1998;11(2):151–70. https://doi.org/10.1080/08916159808946559.
Acknowledgements
Open Access funding provided by the Qatar National Library.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Okonkwo, E.C., Wole-Osho, I., Almanassra, I.W. et al. An updated review of nanofluids in various heat transfer devices. J Therm Anal Calorim 145, 2817–2872 (2021). https://doi.org/10.1007/s10973-020-09760-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-09760-2