Abstract
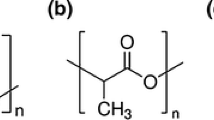
Thermal decomposition of poly(lactic acid) (PLA) has been studied using thermogravimetry coupled to Fourier transform infrared spectroscopy (TGA-FTIR). FTIR analysis of the evolved decomposition products shows the release of lactide molecule, acetaldehyde, carbon monoxide and carbon dioxide. Acetaldehyde and carbon dioxide exist until the end of the experiments, whereas carbon monoxide gradually decreases above the peak temperature in that the higher temperature benefits from chain homolysis and the production of carbon dioxide. A kinetic study of thermal degradation of PLA in nitrogen has been studied by means of thermogravimetry. It is found that the thermal degradation kinetics of PLA can be interpreted in terms of multi-step degradation mechanisms. The activation energies obtained by Ozawa–Flynn–Wall method and Friedman’s method are in good agreement with that obtained by Kissinger’s method. The activation energies of PLA calculated by the three methods are 177.5 kJ mol−1, 183.6 kJ mol−1 and 181.1 kJ mol−1, respectively.






Similar content being viewed by others
References
Chow WS, Lok SK. Thermal properties of polylactides effect of molecular mass and nature of lactide isomer. J Therm Anal Calorim. 2009;95:957–64.
Ahmed J, Zhang JX, Song Z, Varshney SK. Thermal properties of poly(lactic acid)/organo-montmorillonite nanocomposities. J Therm Anal Calorim. 2009;95:627–32.
Drumond WS, Mothé CG, Wang SH. Quantitative analysis of biodegradable amphiphilic poly(L-lactide)-block-poly(ethyleneglycol)-blockpoly(L-lactide) by using TG, FTIR and NMR. J Therm Anal Calorim. 2006;85:173–7.
Martino VP, Ruseckaite RA, Jiménez A. Thermal and mechanical characterization of plasticized poly (L-lactide-co-D,L-lactide) films for food packaging. J Therm Anal Calorim. 2006;86:707–12.
Jamshidi K, Hyon SH, Ikada Y. Thermal characterizaton of polylactides. Polymer. 1988;29:2229–34
McNeill IC, Leiper HA. Degradation studies of some polyesters and polycarbonates-2. Polylactide: degradation under isothermal conditions, thermal degradation mechanism and photolysis of the polymer. Polym Degrad Stab. 1985;11:309–26.
McNeill IC, Leiper HA. Degradation studies of some polyesters and polycarbonates-1. Polylactide: general features of the degradation under programmed heating conditions. Polym Degrad Stab. 1985;11:267–85.
Kopinke FD, Remmler M, Mackenzie K, Moder M. Thermal decomposition of biodegradable polyesters-II. Poly (lactic acid). Polym Degrad Stab. 1996;53:329-42.
Kopinke FD, Mackenzie K. Mechanism aspects of the thermal degradation of poly(lactic acid) and poly (b-hydroxybutyric acid). J Anal Appl Pyrol. 1997;40:43–53.
Cam D, Marucci M. Influence of residual monomers and metals on poly (L-lactide) thermal stability. Polymer. 1997;38:1879–84.
Babanalbandi A, Hill DJT, Hunter DS, Kettle L. Thermal stability of poly(lactic acid) before and after g-radiolysis. Polym Int. 1999;48:980–84.
Aoyagi Y, Yamashita K, Doi Y. Thermal degradation of poly[(R)-3-hydroxybutyrate], poly[ε-caprolactone], and poly[(S)-lactide]. Polym Degrad Stab. 2002;76:53–9.
Fan YJ, Nishidaa H, Shiraib Y. Pyrolysis kinetics of poly (L-lactide) with carboxyl and calcium salt end structures. Polym Degrad Stab. 2003;79:547–62.
Fan YJ, Nishidaa H, Shiraib Y. Thermal stability of poly (L-lactide): influence of end protection by acetyl group. Polym Degrad Stab. 2004;84:143–9.
Fan YJ, Nishidaa H, Shiraib Y. Thermal degradation behaviour of poly(lactic acid) stereocomplex. Polym Degrad Stab. 2004;86:197–208.
Nishidaa H, Mori T, Hoshihara S. Effect of tin on poly(l-lactic acid) pyrolysis. Polym Degrad Stab. 2003;81:515–23.
Liu XB, Zou B, Li WT. Kinetics of thermo-oxidative and thermal degradation of poly(D,L-lactide) (PDLLA) at processing temperature. Polym Degrad Stab. 2006;91:3259–65.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–91.
Flynn JH, Wall LA. A quick, direct method for the determination of activation energy from thermogravimetric data. Polym Lett. 1966;4:323–8.
Chrissafis K, Paraskevopoulos KM, Bikiaris DN. Thermal degradation mechanism of poly(ethylene succinate) and poly(butylene succinate): comparative study. Thermochimica Acta. 2005;435:142–50.
Chrissafis K, Paraskevopoulos KM, Bikiaris DN. Thermal degradation kinetics of the biodegradable aliphatic polyester, poly(propylene succinate). Polym Degrad Stab. 2006;91:60–8.
Corneliu H, Tachita VB, Oana P. Kinetics of thermal degradation in non-isothermal conditions of some phosphorus-containing polyesters and polyesterimides. Eur Polym J. 2007;43:980–8.
Friedman HL. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to phenolic plastic. J Polym Sci Part C. 1964;6:183–95.
Kissinger HE. Variation of peak temperature with heating rate in differential thermal analysis. J Res Nat Bur Stand. 1956;57:217–21.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–12.
Jang BN, Wilkie CA. The thermal degradation of bisphenol A polycarbonate in air. Thermochimica Acta. 2005;426:73–84.
Marcilla A, Gomez-Siurana A, Menargues S. Qualitative study of the evolution of the composition of the gas evolved in the thermal and HY-catalytic oxidative degradation of EVA copolymers. Thermochimica Acta. 2005;438:155–63.
**e W, Gao ZM, Pan WP. Thermal degradation chemistry of alkyl quaternary ammonium montmorillonite. Chem Mater. 2001;13:2979–90.
**e W, Gao ZM, Liu KL. Thermal characterization of organically modified montmorillonite. Thermochimca Acta. 2001;367–368:339–50.
Wang GA, Cheng WM, Tu YL. Characterizations of a new flame-retardant polymer. Polym Degrad Stab. 2006;91:3344–53.
Vyazovkin S, Sbirrazzuoli N. Isoconversional kinetic analysis of thermally stimulated processes in polymers. Macromol Rapid Commun. 2006;27:1515–32.
Zhang J, Zeng JL, Liu YY, Sun LX. Thermal decomposition kinetics of the synthetic complex Pb(1,4-BDC)·(DMF)(H2O). J Therm Anal Calorim. 2008;91:189–93.
Vyazovkin S. Model-free kinetics staying free of multiplying entities without necessity. J Therm Anal Calorim. 2006;83:45–51.
Acknowledgements
The financial support of the Scientific Research Foundation of Hubei Provincial Education Department (No: B200717003) and the Research Foundation of Wuhan University of Science and Engineering in China (No. 20073202) is greatly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zou, H., Yi, C., Wang, L. et al. Thermal degradation of poly(lactic acid) measured by thermogravimetry coupled to Fourier transform infrared spectroscopy. J Therm Anal Calorim 97, 929–935 (2009). https://doi.org/10.1007/s10973-009-0121-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0121-5