Abstract
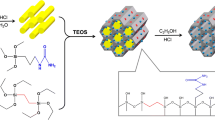
The sorption of uranium(VI) from aqueous solutions was investigated using synthesized magnesium silicate hollow spheres as a novel adsorbent. Batch experiments were conducted to study the effects of initial pH, amount of adsorbent, contact time and initial U(VI) concentrations on uranium sorption efficiency. The desorbing of U(VI) and the effect of coexisting ions were also investigated. Kinetic studies showed that the sorption followed a pseudo-second-order kinetic model. The Langmuir sorption isotherm model correlates well with the uranium sorption equilibrium data for the concentration range of 25–400 mg/L. The maximum uranium sorption capacity onto magnesium silicate hollow spheres was estimated to be about 107 mg/g under the experimental conditions. Desorption of uranium was achieved using inorganic acid as the desorbing agent. The practical utility of magnesium silicate hollow spheres for U(VI) uptake was investigated with high salt concentration of intercrystalline brine. This work suggests that magnesium silicate hollow spheres can be used as a highly efficient adsorbent for removal of uranium from aqueous solutions.










Similar content being viewed by others
References
Tamada M, Seko N, Yoshii F (2004) Radiat Phys chem 71:221–225
Kumar SA, Shenoy NS, Pandey S, Sounderajan S, Venkateswaran G (2008) Talanta 77:422–426
Das S, Pandey AK, Athawale A, Kumar V, Bhardwaj YK, Sabharwal S, Manchanda VK (2008) Desalination 232:243–253
Zhang A, Uchiyama G, Asakura T (2005) React Funct Polym 63:143–153
Zhang A, Asakura T, Uchiyama G (2003) React Funct Polym 57:67–76
Mellah A, Chegrouche S, Barkat M (2006) J Colloid Interface Sci 296:434–441
Sakaguchi T, Nakajima A (1986) Sep Sci Technol 21:519–534
Benedict B, Pigford TH, Levi HW (1981) Nuclear chemical engineering. McGraw-Hill, New York
Yusan S, Akyil S (2008) J Hazard Mater 60:388–395
Morsy AMA, Hussein AEM (2011) J Radioanal Nucl Chem 288:341–346
Zou W, Zhao L, Han R (2011) J Radioanal Nucl Chem 288:239–249
Olmez Aytas S, Akyil S, Eral M (2004) J Radioanal Nucl Chem 260:119–125
Donat R, Esen K, Cetisli H, Aytas S (2009) J Radioanal Nucl Chem 279:253–261
Mellah A, Silem A, Boualia A, Kada R (1992) Chem Eng Process 31:191–194
Saleem M, Afzal M, Qadeer R, Hanif J (1992) Sep Sci Technol 27:239–253
Medina M, Tapia J, Pacheco S, Espinosa M, Rodriguez R (2010) J Non-cryst Solids 356:383–387
Jung Y, Kim S, Park SJ, Kim JM (2008) Colloid Surf A Physicochem Eng Asp 313–314:292–295
Li YH, Liu FQ, **a B, Du QJ, Zhang P, Wang DC, Wang ZH, **a YZ (2010) J Hazard Mater 177:876–880
Wang JH, Zhang X, Zhang B, Zhao YF, Zhai R, Liu JD, Chen RF (2010) Desalination 259:22–28
Zhuang Y, Yang Y, **ang G, Wang X (2009) J Phys Chem C 113:10441–10445
Wang YQ, Wang GZ, Wang HQ, Liang CH, Cai WP, Zhang LD (2010) Chem Eur J 16:3497–3503
Wang YQ, Wang GZ, Wang HQ, Cai WP, Liang CH, Zhang LD (2009) Nanotechnology 20:155604
Wang YQ, Wang GZ, Wang HQ, Cai WP, Zhang LD (2008) Chem Commun 48:6555–6557
Strandwitz NC, Stucky GD (2009) Chem Mater 21:4577–4582
Venkatathri N (2008) Mater Lett 62:462–465
Donat R (2009) J Chem Thermodyn 41:829–835
Taskaev E, Apostoloz D (1978) J Radioanal Chem 45:65–71
Ho YS, Mckay G (1999) Process Biochem 34:451–465
Langmuir I (1918) J Am Chem Soc 40:1361–1403
Freundlich HM (1906) Z Phys Chem 57:385–470
Humelnicu D, Popovici E, Dvininov E, Mita C (2009) J Radioanal Nucl Chem 279:131–136
Das D, Sureshkumar MK, Koley S, Mithal N, Pillai CGS (2010) J Radioanal Nucl Chem 285:447–454
Kim JH, Lee HI, Yeon JW, Jung Y, Kim JM (2010) J Radioanal Nucl Chem 286:129–133
Acknowledgments
The present work was supported by National Natural Science Foundation of China (Grant no. 20901080, 10905074) and Knowledge Innovation Program of The Chinese Academy of Sciences (Grant No. KJCX2-YW-N50-3, KJCX2-YW-N49-2).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fan, F., Ding, H., Bai, J. et al. Sorption of uranium(VI) from aqueous solution onto magnesium silicate hollow spheres. J Radioanal Nucl Chem 289, 367–374 (2011). https://doi.org/10.1007/s10967-011-1091-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-011-1091-1