Abstract
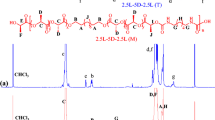
Poly(L-lactide) (PLLA) oligo-esters with α-hydroxyl-ω-alkyl (alkyl = −CH2−[CH2−CH2]m−CH3, where m = 1, 2, 4, 5, 6, 7, 8, 9, and 10) end groups were synthesized by ring-opening polymerization of L-lactide (L-LA) catalyzed by tin(II) 2-ethylhexanoate Sn(Oct)2 in the presence of aliphatic alcohols as initiators (HO−CH2−[CH2−CH2]m−CH3, where m = 1, 2, 4, 5, 6, 7, 8, 9, and 10). High yields (~ 62 to 71%) and M n(NMR) in the range of 2120–2450 Da (PLLA) were obtained. Effects of alkyl end groups on thermal properties of the oligo-esters were analyzed by DSC, TGA and SAXS. Glass transition temperature (T g) gradually decreases with increase in the percent of−CH2−[CH2−CH2]m−CH3 end group, as results alkyl end group provides most flexibility to PLLA. An important effect of alkyl end group on a double cold crystallization (T c1 and T c2) was observed, and is directly related with the segregation phase between alkyl end group and PLLA. TGA analysis revealed that PLLA oligo-esters are more thermally stable with docosyl (−C22H45) respect to the butyl (−C4H9) end group, probably is due to steric hindrance of the end group (docosyl respect to butyl) toward intermolecular and intramolecular transesterification. SAXS analysis showed that alkyl end group as docosyl restricted the growth of lamellae thickness (D) due to steric hindrance. Characterization of hydroxyl and alkyl end groups in the PLLA oligo-esters was determined by MALDI-TOF, GPC, FT-IR and 1 H and 13 C NMR.










Similar content being viewed by others
References
Fukushima K, Kimura Y (2006) Polym Int 55:626–642
Stolt M, Krasowska K, Rutkowska M, Janik H, Rosling A, Södergård A (2005) Polym Int 54:362–368
Korhonen H, Helminen A, Seppälä JV (2001) Polymer 42:7541–7549
Gottschalk C, Frey H (2006) Macromolecules 39:1719–1723
Miola-Delaite C, Hamaide T, Spitz R (1999) Macromol Chem Phys 200:1771–1778
Kowalski A, Duda A, Penczek S (2000) Macromolecules 33:689–695
Fan Y, Chen G, Tanaka J, Tateishi T (2005) Biomacromolecules 6:3051–3056
Spasova M, Mespouille L, Coulembier O, Paneva D, Manolova N, Rashkov L, Dubois P (2009) Biomacromolecules 10:1217–1223
Karanikolopoulos N, Zamurovic M, Pitsikalis M, Hadjichristidis N (2010) Biomacromolecules 11:430–438
Wang Y, Hillmyer MA (2001) J Polym Sci Part A Polym Chem 39:2755–2766
Abayasinghe NK, Glaser S, Prasanna K, Perera U, DWJr S (2005) J Polym Sci Part A Polym Chem 43:5257–5266
Kurokawa K, Yamashita K, Doi Y, Abe H (2006) Polym Degrad Stab 91:1300–1310
Kurokawa K, Yamashita K, Doi Y, Abe H (2008) Biomacromolecules 9:1071–1078
Kobori Y, Iwata T, Doi Y, Abe H (2004) Biomacromolecules 5:530–536
Ouchi T, Ohya Y (2004) J Polym Sci Part A Polym Chem 42:453–462
Storey RF, Sherman JW (2002) Macromolecules 35:1504–1512
Storey RF, Mullen BD, Desai GS, Sherman JW, Tang CN (2002) J Polymer Sciences. Part A: Polymer Chemistry 40:3434–3442
Huang C-H, Wang F-C, Ko B-T, Yu T-L, Lin C-C (2001) Macromolecules 34:356–361
Báez JE, Martínez-Richa A, Marcos-Fernández A (2005) Macromolecules 38:1599–1608
Báez JE, Martínez-Rosales M, Martínez-Richa A (2003) Polymer 44:6767–6772
Finne A, Albertsson A-C (2004) J Polym Sci Part A Polym Chem 42:444–452
Chen H-L, Ko B-T, Huang B-H, Lin C-C (2001) Organometallics 20:5076–5083
Kricheldorf HR, Hachmann-Thiessen H, Schwarz G (2004) Biomacromolecules 5:492–496
Lemmouchi Y, Perry MC, Amass AJ, Chakraborty K, Schué F (2007) J Polym Sci Part A Polym Chem 45:2235–2245
Messman JM, Scheuer AD, Storey RF (2005) Polymer 46:3628–3638
Silverstein RM, Webster FX, Kiemle DJ (2005) Spectrometric identification of organic compounds. John Wiley & Sons, New Jersey
Qian H, Bei J, Wang S (2000) Polym Degrad Stab 68:423–429
Huang M-H, Li S, Coudane J, Vert M (2003) Macromol Chem Phys 204:1994–2001
Takizawa K, Nulwala H, Hu J, Yoshinaga K, Hawker CJ (2008) J Polym Sci. Part A: Polym Chem 46:5977–5990
Gottschalk C, Wolf F, Frey H (2007) Macromol Chem Phys 208:1657–1665
Castillo RV, Müller AJ, Lin M-C, Chen H-L, Jeng U-S, Hillmyer MA (2008) Macromolecules 41:6154–6164
Ring JO, Thomann R, Mülhaupt R, Raquez J-M, Degée P, Dubois P (2007) Macromol. Chem Phys 208:896–902
Jamshidi K, Hyon S-H, Ikada Y (1988) Polymer 29:2229–2234
Acknowledgements
J.E.B. is much indebted to the Instituto de Ciencia y Tecnología de Polímeros (CSIC), Consejo Nacional de Ciencia y Tecnología (CONACYT, México) and Sistema Nacional de Investigadores (SNI, México). J.E.B. and A.M.F thanks to PURAC Biomaterials for the donation of L-lactide (L-LA) monomer. A.M.F. and J.E.B. thanks the Ministerio de Educación y Ciencia for its financial support in accessing the Synchrotron, and François Fauth and Ana Pastor for their help on the beamline BM16 (Grenoble, France). J.E.B. thanks to Jesús L. Pablos and Mario Luzón for obtaining GPC chromatograms.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Báez, J.E., Marcos-Fernández, Á. & Galindo-Iranzo, P. Exploring the effect of alkyl end group on poly(L-lactide) oligo-esters. Synthesis and characterization. J Polym Res 18, 1137–1146 (2011). https://doi.org/10.1007/s10965-010-9517-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10965-010-9517-y