Abstract
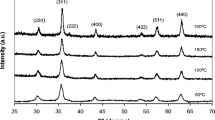
Iron oxide nanoparticles were synthesized by co-precipitation in air atmosphere at different temperatures and their structural and magnetic properties were investigated. The mean particle sizes of iron oxide nanoparticles were calculated from the X-ray diffraction (XRD) patterns using the Scherrer equation. Fourier transform infrared spectroscopy analysis exhibited the vibration bands at 563 cm−1 and 620 cm−1 confirming the formation of Fe3O4 and γ-Fe2O3, respectively. Morphological observation was made by a transmission electron microscope and the particle size of iron oxide nanoparticles was found to be around 9 nm which is consistent with the particle size calculated according to the XRD patterns. It was observed that the intensity of the peaks in the patterns and crystallinity increased as the temperature increased. Magnetization curves showed zero coercivities indicating that the samples are superparamagnetic.
Similar content being viewed by others
References
Lu, A.H., Salabas, E.L., Schüth, F.: Angew. Chem. Int. Ed. 46, 1222 (2007)
Zhou, S.M., Zhao, S.Y., He, L.F., Guo, Y.Q., Shi, L.: Mater. Chem. Phys. 120, 75 (2010)
Fan, R., Chen, X.H., Gui, Z., Liu, L., Chen, Z.Y.: Mater. Res. Bull. 36, 497 (2001)
Aubert, T., Grasset, F., Mornet, S., Duguet, E., Cador, O., Cordier, S., Molard, Y., Demange, V., Mortier, M., Haneda, H.: J. Colloid Interface Sci. 341, 201 (2010)
Kim, D.K., Mikhaylova, M., Zhang, Y., Muhammed, M.: Chem. Mater. 15, 1617 (2003)
Gnanaprakash, G., Mahadevan, S., Jayakumar, T., Kalyanasundaram, P., Philip, J., Raj, B.: Mater. Chem. Phys. 103, 168 (2007)
Liu, Z.L., Liu, Y.J., Yao, K.L., Ding, Z.H., Tao, J., Wang, X.: J. Mater. Synth. Process. 10(2), 83 (2002)
Babes, L., Denizot, B., Tanguy, G., Le Jeune, J.J., Jallet, P.: J. Colloid Interface Sci. 212, 474 (1999)
Namduri, H., Nasrazadani, S.: Corros. Sci. 50, 2493 (2008)
Jain, N., Wang, Y., Jones, S.K., Hawkett, B.S., Warr, G.G.: Langmuir 26(6), 4465 (2010)
Hunt, C.P., Morkowitz, M.B., Banerjee, S.K.: Rock physics and phase relations. In: A Handbook of Physical Constants, p. 196. American Geophysical Union, Washington (1995)
Chantrell, R.W., Popplewell, J., Charles, S.W.: Physica B+C 86–88, 1421 (1977)
O’Grady, K., Bradbury, A.: J. Magn. Magn. Mater 39, 91 (1983)
Kaiser, R., Miskolczy, G.: J. Appl. Phys. 41, 1064 (1970)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karaagac, O., Kockar, H. & Tanrisever, T. Properties of Iron Oxide Nanoparticles Synthesized at Different Temperatures. J Supercond Nov Magn 24, 675–678 (2011). https://doi.org/10.1007/s10948-010-0932-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-010-0932-4