Abstract
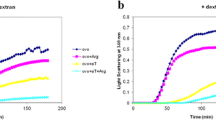
Amyloid fibrils arise from the slow aggregation of intermediately folded protein states. In this study the kinetics of the protein fibril formation of α-lactalbumin and its prevention by αS-casein in the presence and absence of the crowding agent, dextran (68 kDa), have been compared using a thioflavin T binding assay. It was found that αS-casein, a molecular chaperone found in bovine milk, is a potent in vitro inhibitor of α-lactalbumin fibrillization. The effect of αS-casein in preventing fibril formation was significant, although less than it is in the absence of the crowding agent, dextran. The interaction between the chaperone and the α-lactalbumin and structural change in the target protein are also shown using intrinsic fluorescence intensity, an ANS binding assay, CD spectroscopy and size-exclusion HPLC. In summary, α-casein interacts with α-lactalbumin and prevents amyloid formation but not as well as it does when the crowding agent, dextran, not present.






Similar content being viewed by others
Abbreviations
- ThT:
-
Thioflavin T
- ANS:
-
1-anilino-8-naphthalene sulfonic acid
- CD:
-
Circular dichroism
- DTT:
-
Deuterated dithiothreitol
- HPLC:
-
High performance liquid chromatography
References
Acharya K, Ren J, Stuart D, Phillips D (1991) J Mol Biol 221:571–581
Alaimo MH, Farrell HM Jr, Germann MW (1999) Biochim Biophys Acta 1431:410–420
Arrigo AP, Muller WEG (2001) Small stress proteins (WEG Muller (Managing Editor), PJ, I Kostovic Y, Kuchino A, Macieira-coelho RE, Rhoads, Ed)
Bhattacharyya J, Das PK (1999) J Biol Chem 274:15505–15509
Bobe G, Beitz DC, Freeman AE, Lindberg GL (1998) J Agric Food Chem 46:458–463
Bukvist M, Lindstro F, Watts A (2004) J Mol Biol 335:1039–1045
Carver JA, Guerreiro KN, Nicholls A, Truscott RJW (1995) Biochim Biophys Acta 1252:251–260
Carver JA, Lindner RA, Lyon C, Canet D, Hernandez H, Dobson CM, Redfield C (2002) J Mol Biol 318:815–827
Dalgleish DG, Spagnuolo PA, Goff DH (2004) Int Dairy J 14:1025–1031
Ellis RJ (1999) Curr Biol 9:137–139
Ellis RJ (2001) Curr Opin Struct Biol 11:114–119
Engel MF, Van Mierlo CPM, Visser AJWG (2002) J Biol Chem 277:10922–10930
Ewbank JJ, Creighton TE (1993) Biochem 32:3694–3707
Freifelder D (1982) Physical biochemistry-applications to biochemistry and molecular biology (company, W.H.F.a., Ed, Ed), New York
Fox PF, McSweeney PLH (1998) In: Dairy Chemistry and Biochemistry. Blackie Academic & Professional, an imprint of Chapman & Hall, London
Goers J, Permyakov SE, Uversky VN, Fink LA (2002) Biochem 41:12546–12551
Haley DA, Horwitz J, Stewart PL (1998) J Mol Biol 277:27–35
Hatters DM, Lindner RA, Carver JA, Howlett JG (2001) J Biol Chem 276:33755–33761
Hatters DM, Minton AP, Howlett GJ (2002) J Biol Chem 227:7824–7830
Jimenez-Flores MH (2004) J Dairy Sci 87:1641–1674
Kelleher SL, Chatterton D, Nielsen K, Lonnerdal B (2003) Am J Clin Nutr 77:1261–1268
Kruif DCG, May RP (1991) Eur J Biochem 200:431–436
Kuwajima K (1996) FASEB J 10:102–109
Laureto PPVD, Frare E, Gottardo R, Vandael H, Fontana A (2002) Protein Sci 11:2932–2946
Lindner RA, Kapur A, Carver JA (1997) J Biol Chem 272:27722–27729
Lindner RA, Kapur A, Mariani M, Titmuss ST, Carver JA (1998) Eur J Biochem 258:170–183
Lindner RA, Treweek TM, Carver JA (2001) Biochem J 354:79–87
Ohnishi S, Takano K (2004) Cell Mol Life Sci 61:511–524
Rekas A, Adda GC, Aquilina JA, Barnham JK, Sunde M, Galatis D, Williamson AN, Masters LC, Anders FR, Robinson VC, Cappai R, Carver JA (2004) J Mol Biol 340:1167–1183
Ren J, Stuart D, Acharya K (1993) J Biol Chem 268:19292–19298
Sasahara K, MacPhee P, Minton AP (2003) J Mol Biol 326:1227–1237
Swaisgood HE (1992) Chemistry of the caseins in Advanced Dairy Chemistry-1: protein, 2nd edn. Elsevier Applied Science, London, pp 63–110
Serpell LC, Sunde M, Fraser PE, Luther PK, Morris EP, Sangren O, Lundgren E, Blake CC (1995) J Mol Biol 254:113–118
Eftink RM (1994) Biophys J 66:482–501
Thorn CD, Ecroyd H, Sunde M, Poon S, Carver JA (2008) Biochem 47:3926–3996
Thorn D, Meehan S, Sunde M, Rekas A, Gras LS, MacPhee EC, Dobson MC, Wilson RM, Carver JA (2005) Biochem 44:17027–17036
van den Berg B, Ellis RJ, Dobson CM (1999) EMBO J 18:6927–6933
Wetzel R (2002) Struct 10:1031–1106
Zimmerman SB, Minton AP (1993) Annu Rev Biophys Biomol Struct 22:27–65
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghahghaei, A., Divsalar, A. & Faridi, N. The Effects of Molecular Crowding on the Amyloid Fibril Formation of α-Lactalbumin and the Chaperone Action of α-Casein. Protein J 29, 257–264 (2010). https://doi.org/10.1007/s10930-010-9247-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-010-9247-3