Abstract
A new monomer has been synthesized on the basis of vanillin. This compound has the advantage of having a tertiary amine group, which makes it one of the pH-responsive monomers. Moreover, the active aldehyde group facilitates the additional reaction modification like the post-polymerization through Schiff reaction by reaction with primary amine compounds like amino acids. The name of the compound is 2-((dimethylamino)methyl)-4-formyl-6-methoxyphenyl acrylate (DMAMVA). The compounds have been evaluated by 1H, 13C NMR, and FTIR. A new series of polymers has been fabricated by free radical polymerization of N,N-dimethylacrylamide, and 5, 10, and 15 mol% of DMAMVA with N-isopropylacrylamide to produce the dual-responsive thermo-pH polymers. They were reacted with amino acid by post-polymerization according to Schiff base click reaction. All polymers have been investigated chemically in addition to physical characterizations e.g., molecular weight by GPC, glass transition temperature by DSC, chemical decomposition TGA, and morphological by SEM. The study was focused on the determination of phase transition temperatures and LCST of the polymer solution; it has been done using two methods one by UV–VIS-spectroscopy as turbidity test, while the other new technology by micro-DSC. More applications for these polymers will be applied in bio-separation and biotechnology in our future work.
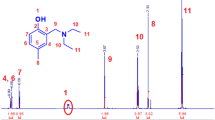
Graphic abstract
















Similar content being viewed by others
References
Tanaka M, Nakahata M, Linke P, Philipp L, Stefan K (2020) Stimuli-responsive hydrogels as a model of the dynamic cellular microenvironment. Polym J 52:861–870. https://doi.org/10.1038/s41428-020-0353-6
Abdelaty MSA (2020) Influence of Vanillin Acrylate and 4-acetylphenyl acrylate hydrophobic functional monomers on phase separation of N-isopropylacrylamide environmental terpolymer: fabrication and characterization. Polym Bull 77:2905–2922. https://doi.org/10.1007/s00289-019-02890-0
Abdelaty MSA (2020) The effect hydrophilic/hydrophobic interaction of 2 ((dimethylamino)methyl) 4 formyl 6 methoxyphenyl acrylate and 4 acetylphenyl acrylate monomers on the phase transition temperature of N isopropylacrylamide terpolymers. J Polym Environ 28:2584–2598. https://doi.org/10.1007/s10924-020-01790-z
Abdelaty MSA (2020) The influence of vanillin acrylate derivative on the phase separation temperature of environmental photo-cross-linked N-isopropylacrylamide copolymer and hydrogel thin films. J Polym Environ. 28:2599–2615. https://doi.org/10.1007/s10924-020-01793-w
Liang H, Qiang Z, Xue L, Michael JS (2019) Stimuli-responsive polymers for sensing and actuation. Mater Horiz 6:1774–1793. https://doi.org/10.1039/C9MH00490D
Seidi F, Jenjob R, Crespy D (2018) Designing smart polymer conjugates for controlled release of payloads. Chem Rev 11:3965–4036. https://doi.org/10.1021/acs.chemrev.8b00006
Chen J-K, Chang C-J (2014) Fabrication and applications of stimuli-responsive polymer films and patterns on surface. Materials 7:805–875. https://doi.org/10.3390/ma7020805
Nayeleh D, Changhe Z, Sarah SK, Angus PRJ, Georgina KS (2019) pH-responsive polymer nanoparticles for drug delivery. Macromol Rapid Commun 40:e1800917. https://doi.org/10.1002/marc.201800917
Mengle K, **nwen P, Hao C, Peiwen L, Bo P, Kai Z (2020) pH-responsive polymeric nanoparticles with tunable sizes for targeted drug delivery. RSC Adv 10:4860–4868
Sijie X, Matthew JW (2020) Temperature-responsive supramolecular hydrogels. J Mater Chem B. https://doi.org/10.1039/D0TB01814G
Liu Z, Zhang S, He B, Shoujuan W, Fangong K (2020) Temperature-responsive hydroxypropyl methylcellulose-N-isopropylacrylamide aerogels for drug delivery systems. Cellulose. https://doi.org/10.1007/s10570-020-03426-w
Tao X, Ting L, Wei-Feng Z, Cheng-Sheng Z (2019) Ionic-strength responsive zwitterionic copolymer hydrogels with tunable swelling and adsorption behaviors. Langmuir 35:1146–1155
Chikara K, Akihiro K, Kenji U, Toshikazu T, Masatoshi K, Kohzo I (2013) Pressure-responsive polymer membranes of slide-ring gels with movable cross-links. Adv Mater 6:4636–4640. https://doi.org/10.1002/adma.201301252
Valentina M, Pierfrancesco C, Marta G, Bartosz T, Veronica A (2017) Light-responsive polymer micro- and nano-capsules. Polymers 9:1–19. https://doi.org/10.3390/polym9010008
Sarah VW, Franka E-R, Dominic B, Didem D, Mathias U (2019) Glucose-responsive polymeric hydrogel materials: from a novel technique for the measurement of glucose binding towards swelling pressure sensor applications. ACS Appl Bio Mater 2:2464–2480
Rostam HM, Leanne EF, Andrew LH, Laurence B et al (2020) Immune-instructive polymers control macrophage phenotype and modulate the foreign body response in vivo. Matter 2:1564–1581
Qiao S, Wang H (2018) Temperature-responsive polymers: synthesis, properties, and biomedical applications. Nano Res 11:5400–5423. https://doi.org/10.1007/s12274-018-2121-x
Abdelaty MSA (2018) Environmental functional photo-cross-linked hydrogel bilayer thin films from vanillin (part 2): temperature responsive layer A, functional, temperature and pH layer B. Polym Bull 11:4837–4858. https://doi.org/10.1007/s00289-018-2297-y
Dirk S (2006) Thermo- and pH-responsive polymers in drug delivery. Adv Drug Deliv Rev 58:1655–1670
Castro-Hernández A, Cortez-Lemus NA (2019) Thermo/pH responsive star and linear copolymers containing a cholic acid-derived monomer, N-isopropylacrylamide and acrylic acid: synthesis and solution properties. Polymers 11:1859. https://doi.org/10.3390/polym11111859
Abdelaty MSA (2018) Poly(N-isopropylacrylamide-co-2-((diethylamino)methyl)-4 formyl-6-methoxyphenylacrylate) environmental functional copolymers: synthesis, characterizations, and grafting with amino acids. Biomolecules 8:138. https://doi.org/10.3390/biom8040138
Abdelaty MSA (2018) Environmental functional photo-cross-linked hydrogel bilayer thin films from vanillin. J Polym Environ 26:2243–2256. https://doi.org/10.1007/s10924-017-1126-y
Abdelaty MSA (2018) Preparation and characterization of new environmental functional polymers based on vanillin and n-isopropylacrylamide for post polymerization. J Polym Environ 26:636–646. https://doi.org/10.1007/s10924-017-0960-2
Weizhong Y, Wen G, Hui Z, Jie R (2013) Tunable thermo-, pH- and light-responsive copolymer micelles. Polym Chem 4:3934–3937. https://doi.org/10.1039/C3PY00478C
Shibayama M, Tanaka T (1993) Volume phase transition and related phenomena of polymer gels. Adv Polym Sci 109:1–62. https://doi.org/10.1007/3-540-56791-7-1
Ying L, Hongmei C, Dian L, Wenxi W, Ye L, Shaobing Z (2015) pH-responsive shape memory poly(ethylene glycol)–poly(ε-caprolactone)-based polyurethane/cellulose nanocrystals nanocomposite. ACS Appl Mater Interfaces 23:12988–12999
Kocak G, Tuncer C, Bütün V (2017) pH-Responsive polymers. Polym Chem 8:144–176. https://doi.org/10.1039/C6PY01872F
Thomas S, Linda S, Mark G, Stephen R (2016) The pH-responsive behaviour of poly(acrylic acid) in aqueous solution is dependent on molar mass. Soft Matter 12:2542. https://doi.org/10.1039/c5sm02693h
Joko S, Alan F, Cahit E (2011) Synthesis and characterization of surface grafted poly(N-isopropylacrylamide) and poly(carboxylic acid)–iron particles via atom transfer radical polymerization for biomedical applications. J Appl Polym Sci 131:40176. https://doi.org/10.1002/app.40176
Adhimoorthy P, Hsieh-Chih T, Ging-Ho H (2018) Formulation and evaluation of epinephrine-loaded poly (acrylic acid-co-N-isopropylacrylamide) gel for sustained ophthalmic drug delivery. React Funct Polym 124:40–47. https://doi.org/10.1016/j.reactfunctpolym.2018.01.001
Panagiotis GF, Vincent L, Bruno A (2021) Synthesis, aqueous solution behavior and self-assembly of a dual pH/thermo-responsive fluorinated diblock terpolymer. Polym Chem 12:277–290. https://doi.org/10.1039/D0PY01515F
Yang L, Zhaohui W, Qi W, Min L, Gang H, Baran DS, **ming G (2016) Non-covalent interactions in controlling pH-responsive behaviors of self-assembled nanosystems. Polym Chem 7:5949–5956. https://doi.org/10.1039/C6PY01104G
Zefeng S, Ke W, Chengqiang G, Shuang W, Wangqing Z (2016) A new thermo-, pH-, and CO2-responsive homopolymer of poly[N-[2-(diethylamino)ethyl]acrylamide]: is the diethylamino group underestimated? Macromolecules 49:162–171
Aditya J, Nandi D, Chester A, Marie M (2018) Study of poly(N-isopropylacrylamide-co-acrylic acid) (pNIPAM) microgel particle induced deformations of tissue-mimicking phantom by ultrasound stimulation. Langmuir 34:1457–1465. https://doi.org/10.1021/acs.langmuir.7b02801
Benrebouh A, Avoce D, Zhu XX (2001) Thermo- and pH-sensitive polymers containing cholic acid derivatives. Polymer 42:4031–4038. https://doi.org/10.1016/S0032-3861(00)00837-5
Yunxiang S, Feng D (2020) Thermo- and pH-responsive fibrillization of squid suckerin A1H1 peptide. Nanoscale 12:6307–6317. https://doi.org/10.1039/C9NR09271D
Schmaljohann D (2006) Thermo- and pH-responsive polymers in drug delivery. Adv Drug Deliv Rev 58:1655–1670. https://doi.org/10.1016/j.addr.2006.09.020
Angela S, Mikhail S, Enrico F (2020) A thermo-responsive, self-assembling biointerface for on demand release of surface-immobilised proteins. Biomater Sci 8:2673. https://doi.org/10.1039/C9BM01957J
Bijari Anil K, Rati Ranjan N (2019) supramolecular phenoxy-alkyl maleate-based hydrogels and their enzyme/pH-responsive curcumin release. New J Chem 43:5559. https://doi.org/10.1039/C8NJ05796F
Mahmoud N, Mohaddeseh S, Siavash I, Rajender SV (2021) Starch, cellulose, pectin, gum, alginate, chitin and chitosan derived (nano) materials for sustainable water treatment: a review. Carbohydr Polym 251:116986. https://doi.org/10.1016/j.carbpol.2020.116986
Maxence F, Bernard B, Sylvain C (2015) Vanillin, a key-intermediate of biobased polymers. Eur Polym J 68:488–502. https://doi.org/10.1016/j.eurpolymj.2015.03.050
Seong HG, Ryu J, Qian Y et al (2019) Novel hierarchically porous melamine-vanillin polymer: synthesis and application for the Pb(II) ion removal in wastewater. Macromol Res 27:882–887. https://doi.org/10.1007/s13233-019-7121-5
Abdelaty MSA, Kuckling D (2016) Synthesis and characterization of new functional photo cross-linkable smart polymers containing vanillin derivatives. Gels 2:1–13. https://doi.org/10.3390/gels2010003
Abdelaty MSA (2019) Layer by layer photo-cross-linked environmental functional hydrogel thin films based on vanillin: part 3. J Polym Environ 27:1212–1225. https://doi.org/10.1007/s10924-019-01421-2
Acknowledgments
We would like to thank the University of Paderborn.
Funding
There is no funding to report for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdelaty, M.S.A. A Facile Method for the Preparation of Hydrophilic-Hydrophobic Functional Thermo-pH Responsive Terpolymers Based on Poly (NIPAAm-co-DMAA-co-DMAMVA) and Post-polymerization. J Polym Environ 29, 3227–3241 (2021). https://doi.org/10.1007/s10924-021-02117-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-021-02117-2