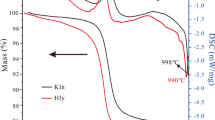
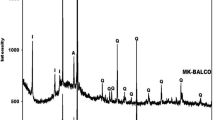
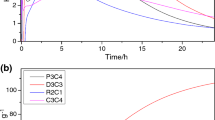
Abstract
This work aims to study the thermal behavior of basic-geopolymers derived from metakaolin (clay). The geopolymers were characterized by different techniques: thermal analysis (DTA, TGA), X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR) and impedance spectroscopy. Some physicochemical properties of the products were also determined: the phases obtained after geopolymer heat treatment and their electrical properties. The results obtained after drying and heat treatment showed that the products kept their initial shapes, but revealed variable colors depending on the temperatures at which they were treated. The products obtained are amorphous between 300 up to 600 °C with peaks relating to the presence of nanocrystallites of muscovites and zeolite, thus at 900 °C it is quite amorphous but only contains nanocrystallites of muscovites. From the temperature of 950 °C, we notice that the geopolymer has been transformed into a crystalline compound predominated by the Nepheline (NaAlSiO4) with the presence of a crystalline phase by minor peaks of Muscovite, this crystalline character has been increased at 1100 °C to obtain a whole phase crystalline of a Nepheline. The treatment of this geopolymer for one hour at 1200 °C shows an amorphous phase again corresponding to corundum (α-Al2O3). This indicates that the dissolution of the grains by the liquid phase induces the conversion of the material structure from sialate [–Si–O–Al–O] to sialate siloxo [–Si–O–Al–O–Si–O–] and the formation of a new crystalline phase (α-Al2O3). This development of sialate to sialate-siloxo was confirmed by IR spectroscopy. As mentioned above, from 300 to 900 °C, Na-sialate geopolymer exhibits the same disorder structure of nepheline. The crystal structure of nepheline is characterized by layers of six-membered tetrahedral rings of exclusively oval conformation. The rings are built by Regularly alternating tetrahedral AlO4 and SiO4. Stacking the layer’s parallel to the c axis gives a three-dimensional network containing channels occupied by Na cations. This topology favors easy movement of Na+ ions throughout the structure. For this reason, ionic migration in nepheline is widely reported. The refinement of Na-Sialate geopolymer at room temperature gives bulk high ionic conductivity of about 5 × 10−5 S cm−1 and this is due to the probable joint contribution of H+ and Na+ ions. Above 200 °C, Na+ seems to remain the only charge carrier with a low activation energy of about Ea = 0.26 eV. At higher temperatures, the characteristic frequencies become so close that it is impossible to distinguish the contributions. A total resistance comprising both grain and grain boundaries contribution is then determined.










Similar content being viewed by others
References
Z. Xu, Z. Huang, C. Liu, H. Deng, X. Deng, D. Hui, X. Zhang, Z. Bai, Nanotechnol. Rev. 10, 779–792 (2021)
S. Zinatloo-Ajabshir, Z. Salehi, M. Salavati-Niasari, J. RSC Adv. 6, 107785–107792 (2016)
Z. Salehi, S. Zinatloo-Ajabshir, M. Salavati-Niasari, J. RSC Adv. 6, 26895–26901 (2016)
S. Zinatloo-Ajabshir, S. Mortazavi-Derazkola, M. Salavati-Niasari, J. Mater. Sci. Mater. Electron. 28, 17849–17859 (2017)
K. Mahdavi, S. Zinatloo-Ajabshir, Q.A. Yousif, M. Salavati-Niasari, Ultrason. Sonochem. 82, 105892 (2022)
S. Zinatloo-Ajabshir, M. Baladi, O. Amiri, M. Salavati-Niasar, J. Sep. Purif.Technol. 248, 117062 (2020)
A. Zonarsaghar, M. Mousavi-Kamazani, S. Zinatloo-Ajabshir, Int. J. Hydrogen Energy 47, 5403–5417 (2022)
S. Zinatloo-Ajabshir, M. Sadat Morassaei, O. Amiri, M. Salavati-Niasari, L. Kok Foong, J. Inorg. Chem. Commun. 136, 109144 (2022)
S. Milad Tabatabaeinejad, S. Zinatloo-Ajabshir, O. Amiri, M. Salavati-Niasari, J. RSC Adv. 11, 40100–40111 (2021)
S. Zinatloo-Ajabshir, M. Salavati-Niasari, J. Int. Appl. Ceram. Technol. 13, 108–115 (2016)
S. Zinatloo-Ajabshir, M. Salavati-Niasari, J. Mater. Sci.: Mater. Electron. 27, 3918–3928 (2015)
S. Hanjitsuwan, P. Chindaprasirt, K. Pimraksa, Int. J. Miner. Metall. Mater. 18, 94 (2011)
J. Davidovits, J. Therm. Anal. 37, 1633–1656 (1991)
A. Palomo, M.T. Blanco-Varela, M.L. Granizo, F. Puertas, T. Vazquez, M.W. Grutzeck, J. Cem. Concr. Res. 29, 997–1004 (1999)
J. Davidovits, J. Therm. Anal. Calorim. 37, 1633–1656 (1991)
J. Davidovits, World Resour. Rev. 6, 263–278 (1994)
J. Davidovits, Geopolymer, Green Chemistry and Sustainable Development Solutions (Geopolymer Institute, Saint-Quentin, 2005)
H. Xu, J.S.J. Van Deventer, Int. J. Miner. Process. 59, 247–266 (2000)
H. Xu, J.S.J. Van Deventer, J. Coll. Surf. A Physicochem. Eng. Aspects 216(15), 27–44 (2003)
H. Xu, J.S.J. Van Deventer, J. Miner. Eng. 15(12), 1131–1139 (2002)
J.G.S. Van Jaarsveld, J.S.J. Van Deventer, L. Lorenzen, J. Miner. Eng. 10, 659–669 (1997)
P. Duxson, G.C. Lukey, J.S.J. van Deventer, J. Mater. Sci. 42, 3044–3054 (2007)
P. Duxson, G.C. Lukey, J.S.J. van Deventer, J. Non-Cryst. Solids 352, 5541–5555 (2006)
A. Mogus-Milankovic, K. Sklepi, P. Mošner, L. Koudelka, P. Kalenda, J. Phys. Chem. 120, 3978 (2016). https://doi.org/10.1021/acs.jpcb.6b01424
Rodriguez-Carvajal, Program FULLPROF, Version 2.6.1 (2005)
G. Varga, J. Pítőanyag. 59, 1 (2007)
R.L. Frost, S.J. van der Gaast, J. Clay Miner. 32, 471–484 (1997)
M.-C. Jodin-Caumon, B. Humbert, N. Phambu, F. Gaboriaud, J. Spectrochim. Acta A: Mol. Biomol. Spectrosc. 72, 959–964 (2009)
C.T. Johnston, J. Helsen, A. Schoonheydt, D.L. Bish, S.F. Agnew, J. Am. Mineral. 83, 75 (1998)
E. Horváth, J. Kristóf, R.L. Frost, J. Princ. Methods Appl. 45, 130–147 (2010)
R.L. Frost, J. Kristof, J.M. Schmidt, J.T. Kloprogge, J. Spectrochim. Acta A: Mol. Biomol. Spectrosc. 57, 603–609 (2001)
V.F.F. Barbosa, K.J.D. Mackenzie, C. Thaumaturgo, Int. J. Inorg. Mater. 2, 309–317 (2000)
U. Rattanasak, P. Chindaprasirt, J. Miner. Eng. 22, 1073–1078 (2009)
C.A. Rees, J.L. Provis, G.C. Lukey, J.S.J. van Deventer, J. Langmuir 23, 9076–9082 (2007)
A. Agarwal, M. Tomozawa, J. Non-Cryst. Solids 209, 166–174 (1997)
M. Sitarz, M. Handke, W. Mozgawa, J. Spectrochim. Acta A 56, 1819–1823 (2000)
S. Peng, L.D. Zhang, G.W. Meng, X.F. Wang, Y.W. Wang, C.Z. Wang, J. Phys. Chem. B 106, 11163–11167 (2002)
V.K. Ahlenberg, H. Böhm, J. Am. Mineral. 83, 631–637 (1998)
G. Roth, H. Böhm, J. Solid State Ion. 19, 553–556 (1986)
A. Jones, D. Palmer, M.S. Islam, M. Mortimer, J. Phys. Chem. Miner. 28, 28–34 (2001)
J. Marcial, J. Kabel, M. Saleh, N. Washton, Y. Shaharyar, A. Goel, J.S. McCloy, J. Am. Ceram. Soc. 101, 2840 (2018)
Acknowledgements
The authors would like to thank the Professor Mohamed Toumi, for the beneficial discussions, and staff of ‘Le Mans Institute of Molecules and Materials (IMMM), CNRS UMR 6283, Le Mans University, avenue Olivier Messiaen, F-72085 LE MANS Cedex 9, France’
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Souissi, F.Z., Hajji, M., Ettoumi, H. et al. Synthesis, Thermal Properties and Electrical Conductivity of Na-Sialate Geopolymer. J Inorg Organomet Polym 32, 3083–3092 (2022). https://doi.org/10.1007/s10904-022-02337-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-022-02337-6